 Multiple Choice Questions
Multiple Choice QuestionsThe bond angle in NF3 (102.3) is smaller than NH4 (107.2). This is because of
large size of F compared to H
large size of N compared to F
opposite polarity of N in the two molecules
small size of H compared to N
The structure of XeF6, is experimentally determined to be distorted octahedron. Its structure according to VSEPR theory is
octahedron
trigonal bipyramid
pentagonal bipyramid
tetragonal bipyramid
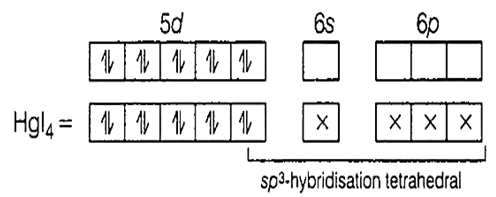
Addition of excess potassium iodide solution to a solution of mercuric chloride gives the halide complex
tetrahedral K2[HgI4]
trigonal K [HgI3]
linear Hg2I2
square planar K2[HgCl2I2]
A.
tetrahedral K2[HgI4]
HgCl2 + 4KI → K2[HgI4] + 2KCl
Hg = [Xe]4f14, 5d106s2
Hg2+ = [Xe]4f14, 5d10, 6s°
The increasing order of O - N - O bond angle in the species NO2, NO2+ and NO2- is
NO2- < NO2 < NO2+
NO2 < NO2- < NO2+
NO2+ < NO2- < NO2
NO2 < NO2+ < NO2-
For BCl3, AlCl3 and GaCl3 the increasing order of ionic character is
BCl3 < AlCl3 < GaCl3
GaCl3 < AlCl3 < BCl3
BCl3 < GaCl3 < AlCl3
AlCl3 < BCl3 < GaCl3
The paramagnetic behaviour of B2 is due to the presence of
two unpaired electrons in MO
two unpaired electrons in MO
two unpaired electrons in MO
two unpaired electrons in MO
CO is practically non-polar since
the -electron drift from C to O is almost nullified by the -electron drift from O to C
the -electron drift from O to C is almost nullified by the -electron drift from C to O
the bond moment is low
there is a triple bond between C and O
The state of hybridization of the central atom and the number of lone pairs over the central atom in POCl3 are
sp,0
sp2, 0
sp3, 0
dsp2, 1
