 Multiple Choice Questions
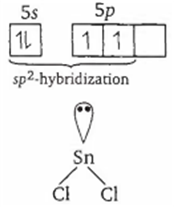
Multiple Choice QuestionsThe atomic number of Sn is 50. The shape of gaseous SnCl2 molecule is
Cl-Sn-Cl



D.

In SnCl2, Sn is sp2 hybridized. As such it has two bond pairs and one lone pair of electrons. Therefore, its structure is as :
The central carbon atom of a free radical contains
6 electrons
7 electrons
8 electrons
10 electrons
Reaction for the formation of NaCl is
Na(g) + Cl2(g) → NaCl(s)
Na(s) + Cl2(g) → NaCl(s)
Na(g) + Cl2(g) → NaCl(s)
Na(g) + Cl2(g) → NaCl(g)
