 Multiple Choice Questions
Multiple Choice QuestionsThe calculated magnetic moment (in Bohr magnetons of Cu2+ ion is)
1.73
zero
2.6
3.4
A.
1.73
Electronic configuration of Cu2+ is-
1s2, 2s2, 2p6, 3s2, 3p6, 3d9 (because 2e- goes from 4s orbital)
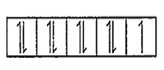
Number of unpaired electrons = 1
Magnetic moment =
=
=
= 1.732
Therefore, 1.732 is the magnetic moment of Cu2+.
Which one of the following molecules contain both ionic and covalent bonds?
CH2Cl2
K2SO4
BeCl2
SO2
What is the hybridization state of the central atom in the conjugate base of NH ion?
sp
sp3
sp2
dsp2
If the bond length and dipole moment of a diatomic molecule are 1.25 A and 1.0 D respectively, what is the percent ionic character of the bond ?
10.66
12.33
16.66
19.33
Which of the following is not correct regarding the properties of ionic compounds?
Ionic compounds have high melting and boiling points
Their reaction velocity in aqueous medium is very high
Ionic compounds in their molten and aqueous solutions do not conduct electricity
They are highly soluble in polar solvents
Match the following lists:
| List - I | List - II |
| A. Ethane | 1. sp carbons |
| B. Ethylene | 2. 6 sp2 carbons |
| C. Acetylene | 3. 2 sp3 carbons |
| D. Benzene |
4. 2 sp2 carbons 5. 1 sp and 1 sp2 carbons |
A - 3; B - 4; C - 1; D - 2
A - 4; B - 5; C - 3; D - 2
A - 3; B - 1; C - 2; D - 5
A - 2; B - 3; C - 4; D - 5
Which of the following pair of ions have same paramagnetic moment?
Cu2+, Ti3+
Mn2+, Cu2+
Ti4+, Cu2+
Ti3+, Ni2+
Average C-H bond energy is 416 kJ mol-1. Which of the following is correct?
CH4 (g) + 416 kJ → C (g) + 4H (g)
CH4 (g) → C (g) + 4H (g) + 416 kJ
CH4 (g) + 1664 kJ → C (g) + 4H (g)
CH4 (g) → C (g) + 4H (g) + 1664 kJ
