Multiple Choice Questions
Multiple Choice QuestionsWhich of the following combination will form an electrovalent bond ?
P and Cl
NH3 and BF3
H and Ca
H and S
C-H bond energy is about 101 kcal/ mol for methane, ethane and other alkanes but is only 77 kcal/ mol for C-H bond of CH3 in toluene. This is because :
of inductive effect due to -CH3 in toluene
of the presence of benzene ring in toluene
of resonance among the structures of benzyl radical in toluene
aromaticity of toluene
Which one of the following compounds has the smallest bond angle in its molecule ?
SO2
OH2
SH2
NH3
All bond angles are exactly equal to 109° 28' in:
methyl chloride
iodoform
chloroform
carbon tetrachloride
The molecular shapes of SF4, CF4 and XeF4 are
different with 1, 0 and 2 lone pairs of electrons on the central atom, respectively
different with 0, 1 and 2 lone pairs of electrons on the central atom, respectively
the same with 1, 1 and 1 lone pair of electrons on the central atoms, respectively
the same with 2, 0 and 1 lone pairs of electrons on the central atom, respectively
Which one of the following does not have sp2 hybridised carbon ?
Acetone
Acetic acid
Acetonitrile
Acetamide
Among the following, the pair in which the two species are not isostructural, is
SiF4 and SF4
IO and XeO3
BH
PF and SF6
A.
SiF4 and SF4
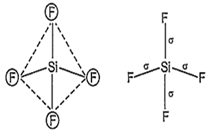
SiF4 and SF4 are not isostructural because SiF4 is tetrahedral due to sp3-hybridisation of Si.
14Si = 1s2, 2s2, 2p6, 3s2 3p2 (In ground state)
14Si = 1s2, 2s2, 2p6, 3s1 3p3 (In excited state)
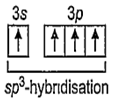
Hence, four equivalent sp3-hybrid orbitals are obtained and they are overlapped by four
p-orbitals of four fluorine atoms on their axes. Thus, it shows following structure:
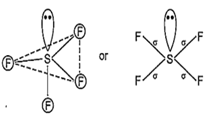
While SF4 is not tetrahedral but it is distorted tetrahedral because in it S is sp3d hybrid and has a lone pair of electron.
16S = 1s2, 2s2 2p6, 3s23p (In ground state)
= 1s2, 2s2 2p6, (In first excitation state)
Hence, sp3d hybrid orbitals are obtained. One orbital is already paired and rest four are overlapped with four p-orbitals of four fluorine atoms on their axes in trigonal bipyramidal form. This structure is distorted from trigonal bi-pyramidal to tetrahedral due to involvement of repulsion between lone pair and bond pair.