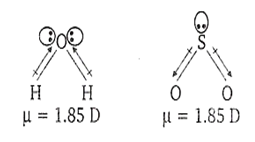Multiple Choice Questions
Multiple Choice QuestionsThe expression for the solubility product of Ag2CO3 will be
Ksp = s2
Ksp = 4s3
Ksp = 27s4
Ksp = s
The correct order towards bond angle is
sp3 < sp2 < sp
sp < sp2 < sp3
sp < sp3 < sp2
sp2 < sp3 < sp
Which of the following has a bond formed by overlap of sp-sp3 hybrid orbitals?
CH3 - C C - H
CH3 - CH = CH - CH3
CH2 = CH - CH = CH2
HC CH
The bond length of HCl bond is 2.29 x 10-10 m. The percentage ionic character of HCl, if measured dipole moment is 6.226 x 10-30 C- m, is
8%
20%
17%
50%
What is the wavelength (in m) of a particle of mass 6.62 x 10-29 g moving with a velocity of 103 ms-1?
6.62 x 10-4
6.62 x 10-3
10-5
105
H-O-H bond angle in H2O is 104.5° and not 109° 28' because of
lone pair-lone pair repulsion
lone pair-bond pair repulsion
bond pair-bond pair repulsion
high electronegativity of oxygen
The atomic number of an element 'M' is 26. How many electrons are present in the M-shell of the element in its M3+ state ?
11
15
14
13
In which of the following pairs, both molecules possess dipole moment ?
CO2, SO2
BCl3, PCl3
H2O, SO2
CO2, CS2
C.
H2O, SO2
Among all the given options, H2O and SO2 both possess dipole moment due to bent structure. Their structure is given below: