 Multiple Choice Questions
Multiple Choice QuestionsThe rate equation for the reaction 2A + B → C is found to be: rate k[A][B]. The correct statement in relation to this reaction is that the
unit of K must be s-1
values of k is independent of the initial concentration of A and B
rate of formation of C is twice the rate of disappearance of A
rate of formation of C is twice the rate of disappearance of A
The soldiers of Napolean army while at Alps during freezing winter suffered a serious problem as regards to the tin buttons of their uniforms. White metallic tin buttons got converted to grey powder. This transformation is related to
an interaction with nitrogen of the air at very low temperatures
an interaction with water vapour contained in the humid air
a change in the partial pressure of oxygen in the air
a change in the partial pressure of oxygen in the air
Consider the following nuclear reactions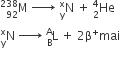
The number of neutrons in the element L is
142
164
144
144
C.
144
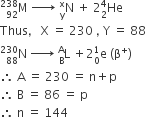
The half-life of a radioisotope is four hours. If the initial mass of the isotope was 200 g, the mass remaining after 24 hours undecayed is
1.042 g
4.167 g
3.125 g
3.125 g
The compound formed in the positive test for nitrogen with the Lassaigne solution of an organic compound is
Fe4[Fe(CN)6]3
Na4[Fe(CN)5NOS]
Fe(CN)3
Fe(CN)3
At 5180C the rate of decomposition of a sample of gaseous acetaldehyde initially at a pressure of 363 Torr, was 1.00 Torr s–1 when 5% had reacted and 0.5 Torr s–1 when 33% had reacted. The order of the reaction is
0
2
3
1
An aqueous solution contains 0.10 M H2S and 0.20 M HCl. If the equilibrium constants for the formation of HS– from H2S is 1.0 x10–7 and that of S2- from HS– ions is 1.2 x 10–13 then the concentration of S2- ions in aqueous solution is
5 x 10-19
5 x 10-8
3 x 10–20
6 x 10-21
For the reaction A +2B → C, the reaction rate is doubled, if the concentration of A is doubled. The rate is increased by four times when concentrations of both A and B are increased by four times. The order of the reaction is
3
0
1
2
The increase in rate constant of a chemical reaction with increasing temperature is (are) due to the fact(s) that
the number of collisions among the reactant molecules increases with increasing temperature
the activation energy of the reaction decreases with increasing temperature
the concentration of the reactant molecules increases with increasing temperature
the number of reactant molecules acquiring the activation energy increases with increasing temperature
The rate of a certain reaction is given by, rate = k [H+]n. The rate increases 100 times when the pH changes from 3 to 1. The order (n) of the reaction is
2
0
1
1.5
