 Multiple Choice Questions
Multiple Choice QuestionsFor the reaction O3(g) + O(g) → 2O2(g), if the rate law expression is, rate = k [O,][O], the molecularity and order of the reaction respectively are
2 and 2
2 and 1.33
2 and 1
1 and 2
The relationship between rate constant and half-life period of zero order reaction is given by
= [A0] 2k
=
Half-life period of a first order reaction, A → product is 6.93 h. What is the value of rate constant?
1.596 h-1
0.1 h-1
4.802 h-1
10 h-1
Which among the following reactions is an example of pseudo first order reaction?
Inversion of cane sugar
Decomposition of H2O2
Conversion of cyclopropane to propene
Decomposition of N2O5
The rate constant for a first order reaction is 7.0 × 10-4 s-1. If initial concentration of reactant is 0.080 M, what is the half life of reaction?
990 s
79.2 s
12375 s
10.10 × 10-4 s
The rate constant of the reaction, 2N2O5 → 4NO2 + O2 at 300 K is 3 × 10-5 s-1. If the rate of the reaction at the same temperature is 2.4 × 10-5 mol dm-3 s-1, then the molar concentration of N2O5 is
0.4 M
0.8 M
0.04 M
0.08 M
In the reaction, A → Products, when the concentration of A was reduced from 2.4 × 10-2 M to 1.2 × 10-2 M, the rate decreased 8 times at the same temperature; The order of the reaction is
0
1
2
3
The half-life period of a first order reaction having rate constant k = 0.231 × 10-10 s-1 will be
32 × 1010 s
2 × 1010 s
3 × 1010 s
2 × 10-10 s
For the reaction X → Y, the concentrations of 'X' are 1.2 M, 0.6 M, 0.3 Mand 0.15 M at 0, 1, 2 and 3 hours respectively. The order of the reaction is
zero
half
one
two
C.
one
For the reaction, concentration of x are
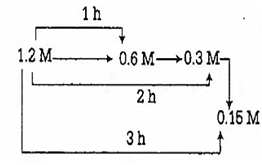
So, half-life(t1/2) = 1h
Therefore, order of the reaction is 'one'.
In a reaction, 2A + B → 3C, the concentration of A decreases from 0.5 mol L-1 to 0.3 mol L-1 in 10 minutes. The rate of production of 'C' during this period is
0.01 mol L-1 min-1
0.04 mol L-1 min-1
0.05 mol L-1 min-1
0.03 mol L-1 min-1
