 Multiple Choice Questions
Multiple Choice QuestionsConsider the following reaction and statements:
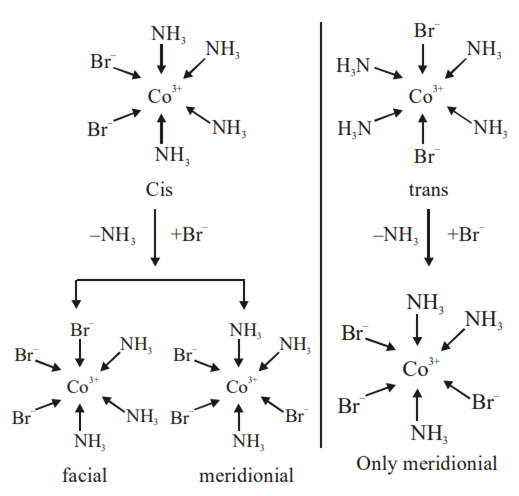
(I) Two isomers are produced if the reactant complex ion is a cis-isomer
(II) Two isomers are produced if the reactant complex ion is a trans-isomer
(III) Only one isomer is produced if the reactant complex ion is a trans-isomer
(IV) Only one isomer is produced if the reactant complex ion is a cis – isomer
The correct statements are
(II) and (IV)
(I) and (II)
(I) and (III)
(III) and (IV)
C.
(I) and (III)
The trans-alkenes are formed by the reduction of alkynes with
Sn - HCl
H2 – Pd/C, BaSO4
NaBH4
Na/liq. NH3
The oxidation states of Cr in [Cr(H2O)6]Cl3,[Cr(C6H6)2], and K2[Cr(CN)2 (O)2(O2)(NH3)] respectively are :
+3, +2, and +4
+3, 0, and +6
+3, 0, and +4
+3, +4, and +6
Amongst [Ni(H2O)6]2+; [Ni(PPh3)2Cl2]; [Ni(CO)4] and [Ni(CN4)]2- the paramagnetic species are
[NiCl4]2-; [Ni(H2O)6]2+; [Ni(PPh3)2Cl2]
[Ni(CO)4]; [Ni(PPh3)2Cl2]; [NiCl4]2-
[Ni(CN4)]2- ; [Ni(H2O)6]2+ ; [NiCl4]2-
[Ni(PPh3)2Cl2] ; [Ni(CO)4]; [Ni(CN4)]2-
Among the following metal carbonyls, the C- O bond order is lowest in
[Mn(CO)6]+
[Fe(CO)5]
[Cr(CO)6]
[V(CO)6]-
Point out incorrect stability order
[Cu(NH3)4]2+ < [Cu(en)2]2+ < [Cu(trien)]2+
[Fe(H2O)6]3+ < [Fe(NO2)6]3- < [Fe(NH3)6]3+
[Co(H2O)6]3+ < [Rh(H2O)6]3+ < [Ir(H2O6]3+
[Cr(NH3)6]+ < [Cr(NH3)6]2+ < [Cr(NH3)6]3+
Which one of the following complexes is not expected to exhibit isomerism?
[Ni(NH3)4 (H2O)2]2+
[Pt(NH3)2Cl2]
[Ni(NH3)2Cl2]
[Ni(em)3]2+
Which of the following complex compounds will exhibit highest paramagnetic behaviour? (At. no. Ti= 22, Cr= 24, Co= 27, Zn= 30)
[Ti(NH3)6]3+
[Cr((NH3)6]3+
[Co(NH3)6]3+
[ZN(NH3)6]2+
Amongst Ni(CO)4, [Ni(CN)4]2- and
Ni(CO)4 and are diamagentic but [Ni(CN)4]2- is paramagnetic.
Ni(CO)4 and [Ni(CN)4]2- are diamagnetic but is paramagnetic.
and [Ni(CN)4]2- are diamagnetic but Ni(CO)4 is paramagnetic.
Ni(CO)4 is diamagnetic but and [Ni(CN)4]2- is paramagnetic.
