 Multiple Choice Questions
Multiple Choice QuestionsThe correct statement with respect to the complexes [Ni(CO)4] and [Ni(CN)4]2- is
nickel is in the same oxidation state in both
both have tetrahedral geometry
both have square planar geometry
have tetrahedral and square planar geometry respectively
The complex ion which has the highest magnetic moment among the following is
[CoF6]3-
[Co(NH3)6]3+
[Ni(NH3)4]2+
[Ni(CN)4]2-
A.
[CoF6]3-
The complex ion which has the highest magnetic moment is [CoF6]3-. In this ion, the oxidation state of cobalt is +3.
Co3+ ion-
![]()
Formation of [CoF6]3+
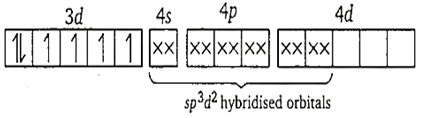
Here, F- ion provides a weak ligand field and is unable to pair up the unpaired electrons of the 3d-orbitals. Thus, it is highly paramagnetic.
When 0.01 mole of a cobalt complex is treated with excess silver nitrate solution, 4.305 g of silver chloride is precipitated. The formula of the complex is
[Co(NH3)3Cl3]
[Co(NH3)5Cl]Cl2
[Co(NH3)6]Cl3
[Co(NH3)4Cl2]NO
Which among the following statements are true for the complex [Co(NH3)6][Cr(CN)6]?
1. It is a non-electrolyte
2. The magnitude of the charge on each complex ion is 3
3. The complex will not conduct current
4. The complex will exhibit coordination isomerism
5. The magnitude of the charge on each complex ion is 1
2 and 4
1 and 4
3 and 5
1 and 2
The primary and secondary valencies of chromium in the complex ion, dichlorodioxalatochromium (III), are respectively
3, 4
4, 3
3, 6
6, 3
(A) K4[Fe(CN)6]
(B) K3[Cr(CN)6]
(C) K3(Co(CN)6]
(D) K2(Ni(CN)4]
Select the complexes which are diamagnetic.
(A), (B) and (C)
(B), (C),and (D)
(A), (C) and (D)
(A), (B) and (D)
Which is not true of the co-ordination compound [Co(en)2Cl2]Cl?
Exhibits geometrical isomerism
Exhibits optical isomerism
Exhibits ionisation isomerism
Is an octahedral complex
Aluminium reacts with NaOH and forms compound 'X'. If the coordination number of aluminiumin 'X' is 6, the correct formula of X is -
[Al(H2O)4(OH)2]+
[Al(H2O)3(OH)3]
[Al(H2O)2(OH)4]-
[Al(H2O)6](OH)3
