 Multiple Choice Questions
Multiple Choice QuestionsThe ratio of anion radius to cation radius of a crystal is 10 : 9.3. Then, the coordination number of the cation in the crystal is
2
6
4
8
Which one of the following gives Prussian blue colour?
Fe2[Fe(CN)6]
Na4[Fe(CN)6]
Fe3[Fe(CN)6]3
Fe4[Fe(CN)6]3
A coordinate complex contains Co3+, Cl- and NH3. When dissolved in water, one mole of this complex gave a total of 3 moles of ions. The complex is
[Co(NH3)6]Cl3
[Co(NH3)5Cl]Cl2
[Co(NH3)4Cl2]Cl
[Co(NH3)3Cl3]
When AgNO3 solution is added in excess to 1M solution of CoCl3. X NH3 one mole of AgCl is formed. What is the value of X?
1
4
3
2
In which of the following coordination compounds, the central metal ion is in zero oxidation state?
[Fe(H2O)6]Cl3
K4[Fe(CN)6]
Fe(CO)5
[Fe(H2O)6]Cl2
Match the following
| Column I | Column II |
| (A) sp3 | (i) [Co(NH3)6]3+ |
| (B) dsp2 | (ii) [Ni(CO)4] |
| (C) sp3d2 | (iii) [Pt(NH3)2Cl2] |
| (D) d2sp3 | (iv) [CoF6]3- |
| (v) [Fe(CO)5] |
A B C D
(v) (ii) (i) (iii)
A B C D
(ii) (iii) (iv) (v)
A B C D
(ii) (iii) (i) (v)
A B C D
(iii) (ii) (iv) (i)
B.
A B C D
(ii) (iii) (iv) (v)
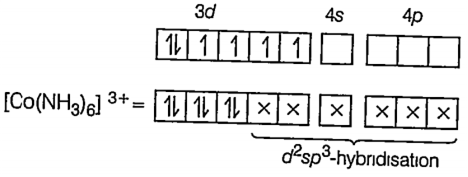
(i) [Co(NH3)6]3+
Here CO is present as CO3+ ion NH3 being strong field ligand pair up the unpaired electron CO.
27CO = [Ar] 3d7 4s2
CO3+ = [Ar] 3d6 4s0
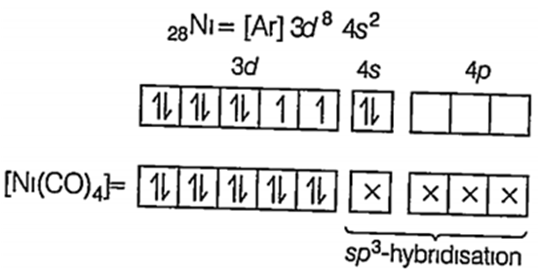
(ii) [Ni(CO)4]
Here Ni is presentin its ground state CO being a strong field ligand, pair up the unpaired electrons of Ni.
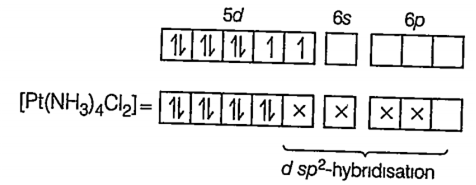
(iii) [Pt(NH3)2Cl2 ]
Here Pt is present as Pt2+ ion.
78Pt = [Xe] 5d9 6s1
Pt2+ = [Xe] 5d8 6s0
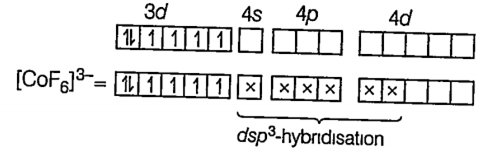
(iv) [CoF6]3-
Here Co is present as Co3+ ion , F being weak field ligand is unable to pair up its unpaired electrons.
Co3+ = [Ar] 3d6 4s0
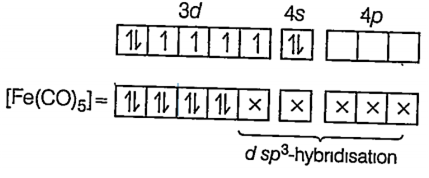
(v) [Fe(CO)5]
Here Fe is present in its ground state.
26Fe = [Ar] 3d6 4s2
Select the correct IUPAC name [Co(NH3)5(CO)3]Cl.
Penta ammonia carbonate cobalt (III) chloride
Pentammine carbonate cobalt chloride
Pentammine carbonato cobalt (III) chloride
Cobalt (III) pentammine carbonate chloride
Identify, from the following, the diamagnetic, tetrahedral complex
[Ni(Cl)4]2-
[Co(C2O4)3]3-
[Ni(CN)4]2-
[Ni(CO)4]
Which type of isomers are [Co(NH3)5Br]SO4 and [Co(NH3)5SO4]Br?
Hydrate isomers
Ionisation isomers
Ligand isomers
Coordination isomers
