 Multiple Choice Questions
Multiple Choice QuestionsAn excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaquachromium (III) chloride. The number of moles of AgCl precipitate would be
0.001
0.002
0.003
0.003
Which of the following acids does not exhibit optical isomerism?
Maleic acid
α-amino acids
Lactic acid
Lactic acid
A.
Maleic acid
Only those compounds exhibit optical isomerism, which has chiral centre and / Or absence of symmetry elements. (chiral carbon is the four valencies of which are satisfied by four different groups.)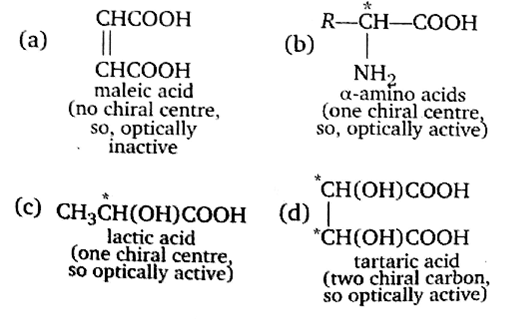
Thus, maleic acid does not exhibit optical isomerism.
Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour?
[Ni(NH3)6]2+
[Zn(NH3)6]2+
[Cr(NH3)6]3+
[Cr(NH3)6]3+
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni (II).Which of the following statements is not true?
Red Complex has a square planar geometry
Complex has symmetrical H- bonding
Red complex has a tetrahedral geometry
Red complex has a tetrahedral geometry
Low spin complex of d6 -cation in an octahedral field will have the following energy
(Δo = Crystal field splitting energy in an octahedral field,
P= electron pairing energy)




The complexes [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6] are the examples of which type of which type of isomerism?
Ionisation isomerism
Coordination isomerism
Geometrical isomerism
Geometrical isomerism
The d- electron configurations of Cr3+, Mn2+ , Fe2+, and Co2+ are d4 ,d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
(At. no. Cr = 24, Mn = 25, Fe =26, Co = 27)
[Fe(H2O)6]2+
[Co(H2O)6]2+
[Cr(H2O)6]2+
[Cr(H2O)6]2+
Of the following complex ions, which is diamagnetic in nature?
[Ni(CN)4]2-
[CuCl4]2-
[CoF6]3-
[CoF6]3-
The sum of coordination number and oxidation number of the metal M in the complex [M(en)2(C2O4)]Cl (where en is ethylenediamine) is
9
6
7
7
