 Multiple Choice Questions
Multiple Choice QuestionsIn [CO(H2O)6]2+, there are three unpaired electrons present. The calculated is 3.87 BM which is quite different from the experimental of 4.40 BM. This is because of
increase in number of unpaired electrons
some contribution of the orbital motion of the electron to the magnetic moment
change in orbital spin of the electron
d-d transition
Anhydrous mixture of KF and HF contain which types of ions?
K+, H+, F-
{KF+, (HF-)}
KH+, F-
K+, H
Magnetic moment of [Ti(H2O)6]4+, [Mn(H2O)6]2+ and [Cr(H2O)6]3+ can be represented as X, Y and Z. They are in order of
X < Z < Y
Z< Y < X
X < Y < Z
Z < Y < X
A chelating agent has two or more than two donor atoms to bind a single metal ion. Which of the following is not a chelating agent?
Thiosulphato
Glycinato
Oxalato
Ethane-1, 2-diamine
The magnetic nature of elements depends on the presence of unpaired electrons. Identify the configuration of transition element, which shows highest magmetic moment
3d7
3d5
3d8
3d2
Which one of the following has a coordinate bond?
NH4Cl
AlCl3
NaCl
Cl2
A.
NH4Cl
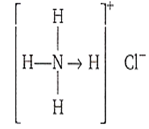
Coordinate bond is present in NH4Cl along with covalent and ionic bonds. The structure of NH4Cl is
Le-blanc process is employed in the manufacture of
baking soda
washing soda
potash
plaster of Paris
The complex ion which has no d-electrons in the central metal atom is
[MnO4]-
[Co(NH3)6]3+
[Fe(CN)6]3-
[Cr(H2O)6]3+
