 Multiple Choice Questions
Multiple Choice QuestionsStandard electrode potential for Sn4+ / Sn2+ couple is +0.15 V and that for the Cr3+/Cr couple is -0.74. These two couples in their standard state are connected to make a cell. The cell potential will be
+0.89 V
+0.18 V
+1.83 V
+1.83 V
A solution contains Fe2+ Fe3+, and I- ions. This solution was treated with iodine at 35o C.Eo for Fe3+/ Fe2+ is +0.77 V and Eo for I2/ 2 I- = 0.536 V. The favourable redox reaction is
I2 will be reduced to I-
There will be No redox reaction
I- will be oxidised to I2
I- will be oxidised to I2
In qualitative analysis, the metals of group I can be separted from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and Pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl- concentration is 0.10 M. What will be the concentration of Ag+ and Pb2+ be at equilibrium? (Ksp for AgCl = 1.8 x 10-10, Ksp for PbCl2 = 1.7 x 10-5)
[Ag+] = 1.8 x 10-7 M; [Pb2+] = 1.7 x 10-6 M
[Ag+] = 1.8 x 10-11 M; [Pb2+] = 8.5 x 10-5 M
[Ag+] = 1.8 x 10-9 M; [Pb2+] = 1.7 x 10-3 M
[Ag+] = 1.8 x 10-9 M; [Pb2+] = 1.7 x 10-3 M
For the reaction of silver ions with copper metal, the standard cell potential was found to be +0.46 V at 25o C. The value of standard Gibbs energy, ΔGo will be (F = 96500 C mol-1 )
-89.0 kJ
-89.0 J
-44.5 kJ
-44.5 kJ
An increase in equivalent conductance of strong electrolyte with dilution is mainly due to
the increase in ionic mobility of ions
100% ionisation of electrolyte at normal dilution
the increase in both, ie, the number of ions and ionic mobility of ions
the increase in both, ie, the number of ions and ionic mobility of ions
A.
the increase in ionic mobility of ions
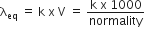
Which of the following expression correctly represents the equivalent conductance at infinite dilution of Al2(SO4)3. Given that  are the equivalent conductance at infinite dilution of the respective ions.
are the equivalent conductance at infinite dilution of the respective ions.




Consider the following relations for emf of an electrochemical cell
A) Emf of cell = (oxidation potential of anode) - (reduction potential of cathode)
B) Emf of cell = (oxidation potential of anode) - ( reducation potential of cathode)
C) Emf of cell = (oxidation potential of anode)- (reduction potential of cathode)
D) Emf of cell = (oxidation potential of anode) - (oxidation potential of anode)-(oxidation potential of cathode)
(C) and (A)
(A) and (B)
(C) and (D)
(C) and (D)
For vaporisation of water at 1 atm pressure, the values of ΔH and ΔS are 40.63 kJ mol- and 108.8 JK-1 mol-1, respectively. The temperature when Gibbs energy change (ΔG) for this transformation will be zero, is
273.14 K
393.4 K
373.4 K
373.4 K
Al2O3 is reduced by electrolysis at low potentials and high currents. If 4.5 x 104 An of current is passed through molten Al2O3 for 6h, what mass of aluminium is produced? (Assume 100% current efficiency, at.mass of Al = 27 g mol-1)
9.0 x 103 g
8.1 x 104 g
2.4 x 105 g
2.4 x 105 g
The equivalent conductance of M/32 solution of weak monobasic acid is 8.0 ohm-cm2 and at infinite dilution is 400 ohm-cm2. The dissociation constant of this acid is
1.25 x 10-5
1.25 x 10-6
6.25 x 10-4
6.25 x 10-4
