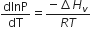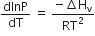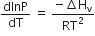Multiple Choice Questions
Multiple Choice QuestionsIn the following compounds, the decreasing order of basic strength will be:
(C2H5)2NH > NH3 > C2H5NH2
(C2H5)2NH > C2H5NH2 > NH3
C2H5NH2 > NH3 > (C2H5)2NH
NH3 > C2H5NH2 > (C2H5)2NH
For the following reactions, equilibrium constans are given:
S(s) + O2(g) SO2(g); K1 = 1052
2S(s) + 3O2(g) SO3(g); K2 = 10129
The equilibrium constant for the reaction,
2SO2(s) + O2(g) SO3(g)
10181
1077
10154
1025
In an acid-base titration, 0.1 M HCl solution was added to the NaOH solution of unknown strength. Which of the following correctly shows the change of pH of the titration mixture in this experiment ?

(D)
(A)
(C)
(B)
Consider the following statements
a) The pH of a mixture containing 400mL of 0.1M H2SO4 and 400 mLof 0.1M NaOH will be approximately 1.3.
b) Ionic product of water is temperature dependent.
c) A monobasic acid with Ka=10-5 has a pH=5. The degree of dissociation of this acid is 50%.
d) The Le Chatelier’s Principle is not applicable to common-ion effect.
The correct statements are
b and c
a,b and d
a,b, and c
a and b
The pH of a 0.02 M NH4Cl solution will be: [ given Kb(NH4OH) = 10-5 and log 2= 0.301]
5.35
2.65
4.65
4.35
For the reaction, 2SO2(g) + O2(g) 2SO3(g), ΔH= -57. 2kJ mol-1 and Kc = 1.7 × 106. Which of the following statement is INCORRECT ?
The equilibrium constant is large suggestive of reaction going to completion and so no catalyst is required
The equilibrium constant decreases as the temperature increases.
The equilibrium will shift in forward direction as the pressure increase
Volume will not affect the equilibrium constant
The addition of a catalyst during a chemical reaction alters which of the following quantities ?
Internal energy
Enthalpy
Activation energy
Activation energy
Which one of the following characteristics is associated with adsorption?
ΔG, ΔH and ΔS all are negative
ΔG and ΔH are negative but ΔS is positive
ΔG and ΔS are negative but ΔH is positive
ΔG and ΔS are negative but ΔH is positive
MY and NY3, two nearly insoluble salts. have the same Ksp values of 6.2 x 10-13 at room temperature. Which statement would be true in regard to MY and NY3 ?
The molar solubility of MY in water is less than that of NY3
The salts MY and NY3 are more soluble in 0.5 M KY than in pure water
The addition of the Salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
The addition of the Salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
Consider the following liquid-vapour equilibrium
Which of the following relations is correct?