Multiple Choice Questions
Multiple Choice QuestionsThe pH of a saturated solution of a metal hydroxide of formula X(OH)2 is 12.0 at 298 K. What is the solubility product of a metal hydroxide at 298 K (in mol3 L-3)?
5 × 10-7
2 × 10-6
1 × 10-7
5 × 10-5
An inorganic salt (A) is decomposed on heating to give two products (B) and (C). Compound (C) is a liquid a room temperature and is neutral to litmus while the compound (B) is a colourless neutral gas. Compounds (A), (B) and (C) are
NH4NO3, N2O, H2O
NH4NO2, NO, H2O
CaO, H2O, CaCl2
Ba(NO3)2, H2O, NO2
The sum of pH and pKb for a basic buffer solution is 13. The ratio of the concentration of the base to that of the salt is
10
1
0.05
0.1
Two equilibria, AB A +B. are simultaneously maintained in a solution with equilibrium constants, K1 and K2 respectively. The ratio of [A+] to [AB] in the solution is
directly proportional to [B-]
inversely proportional to [B-]
directly proportional to the square of [B-]
inversely proportional to the square of [B-]
A current strength of 9.65 A is passed through excess fused AlCl3 for 5 h. How many litres of chlorine will be liberated at STP? (F = 96500 C)
2.016
1.008
11.2
20.16
The colour of the soltuion/ precipitate obtained in the elemental analysis of an organic compound and the molecule/ion responsible for the colour are given below. Choose the incorrectly matched pair.
Prussian blue - Fe4[Fe(CN)6]3.xH2O
Yellow - (NH4)2MoO4
Violet colour - [Fe(CN)5NOS]4-
Blood red colour - [Fe(SCN)]2+
Among the following amines, which one has the highest pK, value in aqueous solution?
Methanamine
N, N-dimethylaniline
Ethanamine
Benzenamine
In the following equilibrium reaction,
2A B + C
the equilibrium concentration of A, B and C are 1 × 10-3 M, 2 × 10-3 M and 3 × 10-3 M respectively at 300 K. The value of Kc for this equilibrium at the same temperature is
6
36
Which one of the following is the correct statement?
HCO is the conjugate base of CO
NH is the conjugate acid of NH3
H2SO4 is the conjugate acid of HSO
NH3 is the conjugate base of NH
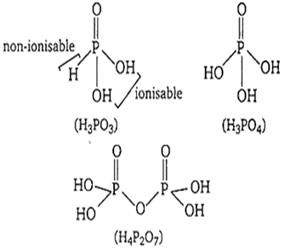
Choose the weak monobasic acid, among the following
H3BO3
H3PO3
H3PO4
HNO3
A.
H3BO3
H3BO3 is a weak Lewis monobasic acid, because it can only accept a pair of electron. None of its hydrogens are ionisable.