 Multiple Choice Questions
Multiple Choice QuestionsThe major products obtained during ozonolysis of 2, 3-dimethyl-1-butene and subsequent reductions with Zn and H2O are
methanoic acid and 2-methyl-2-butanone
methanal and 3-methyl-2-butanone
methanol and 2, 2 -dimethyl-3-butanone
methanoic acid and 2-methyl-3-butanone
1,4-dimethylbenzene on heating with anhydrous AlCl3 and HCl produces
1, 2-dimethylbenzene
1, 3-dimethylbenzene
1,2, 3-trimethylbenzene
ethylbenzene
Best reagent for nuclear iodination of aromatic compounds is
KI/CH3COCH3
I2/CH3CN
KI/ CH3COOH
I2/ HNO3
The IUPAC name of the following molecule is

5,6-dimethylhept-2-ene
2,3-dimethylhept-5-ene
5,6-dimethylhept-3-ene
5-iso-propylhex-2-ene
The order of decreasing ease of abstraction of hydrogen atoms in the following molecules
Ha > Hb > Hc
Ha > Hc > Hb
Hb > Ha > Hc
Hc > Hb > Ha
Reaction of benzene with Me3CCOCl in the presence of anhydrous AlCl3 gives




B.

When benzene reacts with Me3CCOCl in the presence of anhydrous AlCl3 gives the following reaction
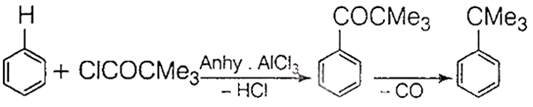
The best method for the preparation of 2, 2-dimethylbutane is via the reaction of
Me3CBr and MeCH2Br in Na/ether
(Me3C)2CuLi and MeCH2Br
(MeCH2)2CuLi and Me3CBr
Me3CMgI and MeCH2I
