 Multiple Choice Questions
Multiple Choice QuestionsObserve the following reactions and predict the nature of A and B:
A and B both are 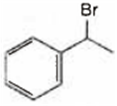
A and B both are ![]()
A is 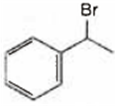 and B is
and B is ![]()
A is ![]() and B is
and B is 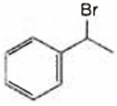
Nitration of aniline in strongly acidic medium, result in the formation of m-nitroaniline also. This is because :
amino group is meta orienting during electrophilic substitution reaction
nitro group goes always to the meta position irrespective of the substituents
nitration of aniline is a nucleophilic substitution reaction in strongly acidic medium
in strongly acidic conditions aniline is present as anilinium ion
Propyne when passed through a hot iron tube at 400°C produces :
benzene
methyl benzene
dimethyl benzene
trimethyl benzene
The name of the compound fig is:
![]()
(2Z, 4Z)-2, 4-hexadiene
(2Z, 4E)-2, 4-hexadiene
(2E, 4Z)-2, 4-hexadiene
( 4E, 4Z)-2, 4-hexadiene
The following reaction represent,
C12H26 → C6H12 + C6H14
substitution
synthesis
cracking
polymerization
Order of reactivity of C2H6, C2H4 and C2H2 is
C2H6 > C2H4 > C2H2
C2H2 > C2H6 > C2H4
C2H4 > C2H2 > C2H6
All are equally reactive.
In Wurtz reaction alkyl halide react with
sodium in ether
sodium in dry ether
sodium only
alkyl halide in ether
B.
sodium in dry ether
In Wurtz reaction alkyl halide react with sodium in dry ether to produce alkane having double number of carbon atoms as in alkyl halide.
2R—X + 2Na R—R + 2NaX
alkyl halid alkane
