 Multiple Choice Questions
Multiple Choice QuestionsIn which of the following reactions, chlorine acts as an oxidising agent?
(i) CH3CH2OH + Cl2 → CH3CHO + HCl
(ii) CH3CHO + Cl2 → CCl3 . CHO + HCl
(iii) CH4 + Cl2 CH3Cl + HCl
The correct answer is-
(i) only
(ii) only
(i) and (iii)
(i), (ii) and (iii)
Which one of the following is an example of disproportionation reaction?
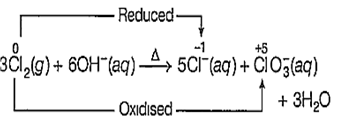
3Cl2 (g) + 6OH- (aq) → ClO (aq) + 5Cl- (aq) + 3H2O (l)
Ag2+ (aq) + Ag (s) → 2Ag+ (aq)
Zn (s) + CuSO4 (aq) → Cu (s) + ZnSO4 (aq)
2KClO3 (s) → 2KCl (s) + 3O2 (g)
A.
3Cl2 (g) + 6OH- (aq) → ClO (aq) + 5Cl- (aq) + 3H2O (l)
A reaction in which the same specie is simultaneously oxidised as well as reduced is called a disproportionation reaction.
One mole of N2H4 loses 10 moles of electrons to form a new compound Z. Assuming that all the nitrogens appear in the new compound, what is the oxidation state of nitrogen in Z? (There is no change in die oxidation state of hydrogen.)
-1
-3
+3
+5
A 100 mL solution of 0.1 N HCl was titrated with 0.2 N NaOH solution.The titration was discontinued after adding 30 mL of NaOH solution. The remaining titration was completed by adding 025 N KOH solution. The volume of KOH required for completing the titration is
16 mL
32 mL
35 mL
70 mL
The gaseous products formed at cathode (X) and anode (Y), when an aqueous solution of sodium acetate is electrolysed are
| X | Y |
| CO2 | C2H6, H2 |
| X | Y |
| H2, CO2 | C2H6 |
| X | Y |
| H2 | C2H6, CO2 |
| X | Y |
| C2H6, H2 | CO2 |
In the chemical reaction,
Cl2 + H2S → 2HCl + S.
The oxidation number of sulphur changes from
0 to 2
2 to 0
-2 to 0
-2 to -1
In the reaction,
3Mg + N2 → Mg3N2
magnesium is reduced
magnesium is oxidised
nitrogen is oxidised
None of the above
When MnO2 is fused with KOH, a coloured compound is formed. The product and its colour is
K2MnO4, purple green
KMnO4, purple
Mn2O3, brown
Mn2O4, black
