Multiple Choice Questions
Multiple Choice QuestionsAir at sea level is dense. This is a practical implimentation of :
Boyle's law
Charles' law
Avogadro's law
Dalton's law
The r.m.s. velocity of CO2 at a temperature T (in kelvin) is x cm s-1. At what temperature (in kelvin), the r.m.s. velocity of nitrous oxide would be 4x cm s-1? (Atomic weights of C, N and O are respectively 12, 14 and 16)
16 T
2 T
4 T
32 T
n moles of an ideal gas at temperature T (in kelvin) occupy V L of volume, exerting a pressure of P atmospheres. What is the concentration (in mol/L) ?
4 g of an ideal gas occupies 5.6035 L of volume at 546 K and 2 atmosphere pressure. What is its molecular weight?
4
16
32
64
If a gas contains only three molecules that move with velocities of 100, 200, 500 ms-1. What is the rms velocity of that gas in ms-1?
100
100√30
100√10
800/3
If the electron of a hydrogen atom is present in the first orbit, the total energy of the electron is
At 27°C, a closed vessel contains a mixture of equal weights of helium (mol. wt. = 4), methane (mol, wt. = 16) and sulphur dioxide (mol. wt. = 64). The pressure exerted by the mixture is 210 mm. If the partial pressures of helium, methane and sulphur dioxide are p1, p2 and p3 respectively, which one of the following is correct?
p3 > p2 > p1
p1 > p2 > p3
p1 > p3 > p2
p2 > p3 > p1
At 27°C, 500 mL of helium diffuses in 30 minutes. What is the time (in hours) taken for 1000 mL of SO2 to diffuse under same experimental conditions?
240
3
2
4
A and B are ideal gases. The molecular weights of A and B are in the ratio of 1 :4. The pressure of a gas mixture containing equal weights of A and B is P atm. What is the partial pressure (in atm) of B in the mixture?
P/5
P/2
P/2.5
3P/4
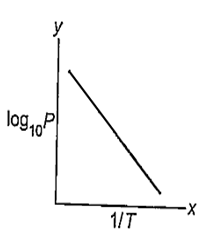
Which of the following set of variables give a straight line with a negative slope when plotted? (P = vapour pressure ; T = Temperature in K)
| y - axis | x - axis |
| P | T |
| log10 P | T |
| log10 P |
| log10 P | log10 |
C.
| log10 P |
Among the given options, option c gives a staright line with a negative slope. It is represented as-
on X-axis and log10 P on Y-axis