 Multiple Choice Questions
Multiple Choice QuestionsA structure of a mixed oxide is cubic close packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is
ABO2
A2BO2
A2B3O4
A2B3O4
The correct statement regarding defects in the crystalline solid is
Schottky defects have no effect on the density of crystalline solid
Frenkel defect decreases the density of crystalline solids
Frenkel defect is a dislocation defect
Frenkel defect is a dislocation defect
A solid compound XY has NaCl structure. If the radius of the cation is 100 pm, the radius of the anion (Y-) will be
275.1 pm
322.5 pm
241.5 pm
241.5 pm
AB crystallises in a body centred cubic lattice with edge length 'a' equal to 387 pm. The distance between two oppositely charged ion in the lattice is
335 pm
250 pm
200 pm
200 pm
Copper crystallises in a face-centered cubic lattice with a unit cell length of 361 pm. What is the radius of copper atom in pm?
128
157
181
108
Lithium metal crystallises in a body centred cubic crystal. If the length of the side of the unit cell lithium is 351 pm, the atomic radius of the lithium will be
240.8 pm
151.8 pm
75.5
75.5
B.
151.8 pm
In case of body centred cubic (bcc) crystal,
Hence, atomic radius of lithium,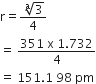
Volume occipied by one molecule of water (density = 1 g cm-3) is
9.0 x 10-23
6.023 x 10-23 cm3
3.0 x 10-23 cm3
3.0 x 10-23 cm3
Which of the following statements is not correct?
The fraction of the total volume occupied by the atoms in a primitive cell is 0.48
molecules solids are generally volatile
the number of carbon atoms in a unit cell
the number of carbon atoms in a unit cell
If 'a' stands for the edge length of the cubic system:simple cubic , body centred cubic and face centred cubic, then the ratio of radii of the spheres in these systems will be repsectively,




