 Multiple Choice Questions
Multiple Choice QuestionsThe product(s) formed when H2O2 reacts with disodium hydrogen phosphate is
P2O5.Na3PO4
Na2HPO4.H2O2
NaH2PO4, H2O
Na2HPO4.H2O
Which one of the following is used in the preparation of cellulose nitrate ?
KNO3
HNO3
KNO2
HNO2
The oxoacid of sulphur which contains two sulphur atoms in different oxidation states is
pyrosulphurous acid
hyposulphurous acid
pyrosulphuric acid
persulphuric acid
Assertion (A) : Noble gases have very low boiling points.
Reason (R) : All noble gases have general electronic configuration of ns2 np6 (except He).
Both (A) and (R) are true and (R) is the correct explanation of (A).
(A) is false but (R) is true.
(A) is true but (R) is false.
Both (A) and (R) are true and (R) is not the correct explanation of (A).
The charring of sugar takes place when treated with concentrated H2SO4. What is the type of reaction involved in it?
Dehydration reaction
Hydrolysis reaction
Addition reaction
Disproportionation reaction
The increasing order of the atomic radius of Si, S, Na, Mg, Al is
S < Si< Al< Mg< Na
Na< Mg < Si< Al< S
Na< Mg< Si< Al < S
Na< Mg< Al< Si< S
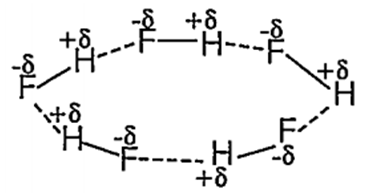
The molecular interactions responsible for hydrogen bonding in HF
ion-induced dipole
dipole-dipole
dipole- induced dipole
ion-dipole
B.
dipole-dipole
Hydrogen bond is simply dipole-dipole attraction within oppositely partially charged ends. In HF molecule, electronegativity difference is remarkable, due to which it behaves as a dipole. In the gaseous state several HF molecules polymerises through H-bonding.
Assertion (A) : AlCl3 exists as a dimer through halogen bridged bonds.
Reason (R) : AlCl3 gets stability by accepting electrons from the bridged halogen.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (H) is false.
(A) is false, but (R) is true.
Which of the following statements regarding sulphur is not correct?
At about 1000 K, it mainly consists of S, molecules.
The oxidation state of sulphur is never less than + 4 in its compounds.
S2 molecule is paramagnetic.
Rhombic sulphur is readily soluble in CS2.
