 Multiple Choice Questions
Multiple Choice QuestionsTotal energy of the electron in hydrogen atom above 0 eV leads to
continuation of energy states
large number of discrete ionised states
balmer series
paschen series
Assertion: Bohr's atomic model cannot be used to explain multiple electron species.
Reason: It does not take inter-electronic interactions in account.
If both assertion and reason are true and reason
is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion: Total energy of electron in an hydrogen atom is negative.
Reason: It is bounded to the nucleus.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
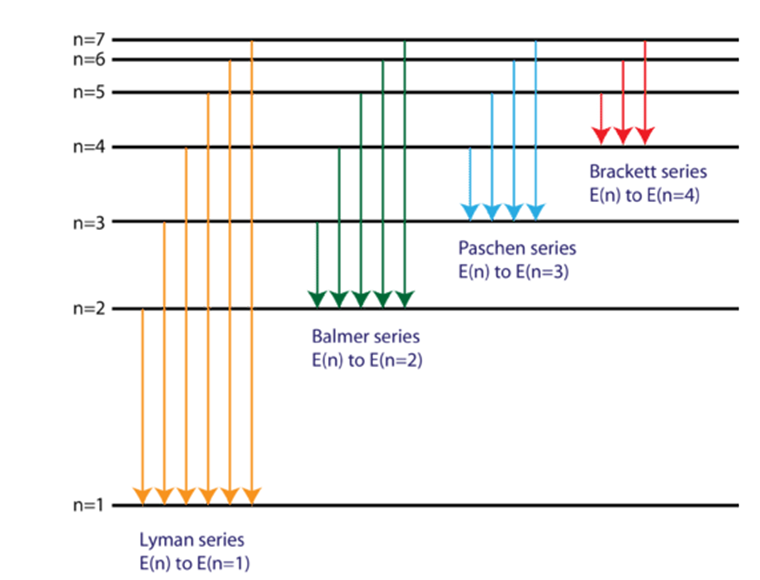
The series corresponding to minimum wavelength transition in H-atom
Balmer series
Lyman series
Paschen series
Brackett series
What would be maximum wavelength for Brackett series of hydrogen-spectrum?
74589 Ao
22790 Ao
40519 Ao
18753 Ao
C.
40519 Ao
For Brackett series
n1 = 4, n2 = 5, 6, 7
For maximum wavelength
n2 = 5
= 1.09687 × 107
λmax = 40519 Ao
The wavelengths of Kα X- rays for lead isotopes Pb208 , Pb206 and Pb204 are λ1, λ2 and λ3 respectively. Then
λ2 =
λ2 = λ1 + λ3
λ2 = λ1 λ2
λ2 =
Assertion: It is essential that all the lines available in the emission spectrum will also be available in the absorption spectrum.
Reason: The spectrum of hydrogen atom is only absorption spectrum.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
