 Multiple Choice Questions
Multiple Choice QuestionsAn ideal monoatomic gas is taken around the cycle ABCDA as shown in the PV diagram. The work done during the cycle is given by

PV
2 PV
4 PV
310 J of heat is required to raise the temperature of 2 moles of an ideal gas at constant pressure from 25°C to 35°C. The amount of heat required to raise the temperature of the gas through the same range at constant volume is
384 J
144 J
276 J
452 J
An ideal gas is taken via path ABCA as shown in figure. The net work done in the whole cycle is
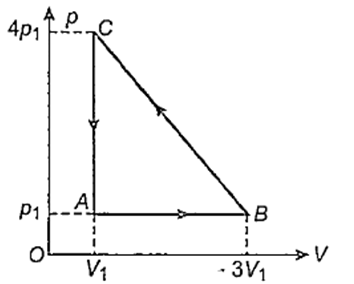
3p1V1
− 3p1V1
6p1V1
Zero
A graph of pressure versus volume for an ideal gas for different processes is as shown. In the graph curve OC represents

isochoric process
isothermal process
isobaric process
adiabatic process
The temperature of a gas contained in a closed vessel of constant volume increases by 1°C when the pressure of the gas is increased by 1%. The initial temperature of the gas is
100 K
273°C
100°C
200 K
The molecules of a given mass of a gas have r.m.s. velocity of 200 m/s at 27o C and 1.0 x 105 Nm-2 pressure. When the temperature and pressure of the gas are respectively, 127o C and 0.05 x 105 Nm-2, the RMS velocity of its molecules in m/s is,




The mean free path of molecules of a gas, (radius r) is inversely proportional to,
r3
r2
r
r
B.
r2
Mean free path, l = 
That is, 
In the given (V-T )diagram, what is the relation between pressures p1 and p2?
p2 =p1
p2>p1
p2<p1
p2<p1
Two vessels separately contain two ideal gases A and B the same temperature, the pressure of A being twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weight of A and B is
2/3
3/4
2
2
