 Multiple Choice Questions
Multiple Choice QuestionsIn a Carnot engine, the temperature of reservoir is 927°C and that of sink is 27°C. If the work done by the engine when it transfers heat from reservoir to sink is 12.6 x 106 J, the quantity of heat absorbed by the engine from the reservoir is
16.8 × 106 J
4 × 106 J
7.6 × 106 J
4.25 × 106 J
In the given p-V diagram, I is the initial state and F is the final state.

The gas goes from I to F by
(i) IAF (ii) IBF (iii) ICF
The heat absorbed by gas is
the same in all three processes
the same in (i) and (ii)
greater in (i) than in (ii)
the same in (i) and (iii)
A heater of 220 V heats a volume of water in 5 min. The same heater when connected to 110 V heats the same volume of water in (minute)
5
20
10
2.5
The p-V diagram of a gas undergoing a cyclic process (ABCDA) is shown in the graph where p is in units of Nm and V in cm. Identify the incorrect statement.
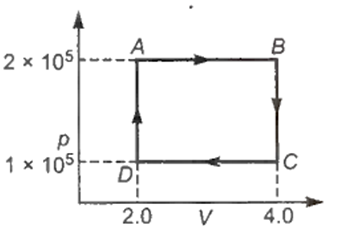
0.4 J of work is done by the gas from A to B.
0.2 J of work is done on the gas from C and D.
Work is done by the gas in going from B to Cand on the gas from D to A.
Net work done by the gas in one cycle is 0.2 J.
A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are (p, 5V) and (l0p, V). If now the piston is left free and the system undergoes isthermal process, then the volume of the gas in two parts respectively are
3 V, 3 V
5 V, V
4 V, 2 V
A.
When the piston is allowed to move, the gases are kept separated but the pressure has to be equal.
The pressure on each side will be
The volume on both side will be adjusted such that the original pressure x volume is kept constant (isothermal change).
On the left hand side,
On the right hand side,
Hence,
A Carnot engine with sink's temprature at 17°C has 50% efficiency. By how much should its source temperature be changed to increase its efficiency to 60%
225 K
128°C
580 K
145 K
Two moles of exygen is mixed with eight moles of helium. The effective specific heat of the mixture at constant volume is
1.3 R
1.4 R
1.7 R
1.9 R
800 cc volume of a gas having is suddenly compressed adiabatically to 100 cc. If the initial pressure is P, then the final pressure will be
8 P
32 P
An ice box made of Styrofoam (Thermal conductivity = 0.01 Jm-1 s-1 K-1) is used to keep liquids cool. It has a total wall area including lid of 0.8 m2 and wall thickness of 2.0cm. A bottle of water is placed in box and filled with ice. If the outside temperature is 30°C, the rate of flow of heat into the box is ( in J s-1)
16
14
12
10
