 Multiple Choice Questions
Multiple Choice QuestionsThermodynamic processes are indicated in the following diagram.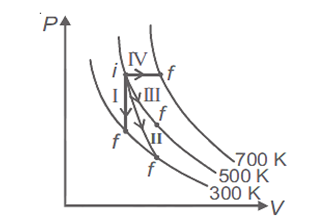
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
P → a, Q → c, R → d, S → b
P → c, Q → a, R → d, S → b
P → c, Q → d, R → b, S → a
P → c, Q → d, R → b, S → a
Carnot engine having an efficiency of 1/10 as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
1 J
90 J
99 J
99 J
A small sphere of radius 'r' falls from rest in a viscous liquid. As a result, heat is produced due to viscous force. The rate of production of heat when the sphere attains its terminal velocity, is proportional to
r3
r2
r4
r5
A sample of 0.1 g of water at 100°C and normal pressure (1.013 × 105 Nm–2) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample, is
104.3 J
208.7 J
84.5 J
42.2 J
B.
208.7 J
Applying first law of thermodynamics equation.
The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is

2/5
2/3
2/7
1/3
The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is
26.8%
20%
12.5%
6.25%
p-V plots for two gases during the adiabatic process as shown in figure plots 1 and 2 should correspond respectively to

He and O2
O2 and He
He and Ar
O2 and N2
The temperature of source and sink of a heat engine are 127oC and 27oC, respectively. An inventor claims its efficiency to be 26%, then
It is impossible
It is possible with high probability
It is possible with low probability
Data are insufficient
Air is expanded from 50 litres to 150 litres at atmosphere the external work done is (1 atmospheric pressure =1)
200J
2000J
Helium at 270C has a volume 8 litres. it is suddenly compressed to a volume of 1 litre. The temperature of the gas will be
93270C
12000C
9270C
1080C
