Multiple Choice Questions
Multiple Choice QuestionsWhich of the following amines gives yellow oily liquid with HNO3?
Ethyl methyl amine
Aniline
3-methyl benzyl amine
Methyl amine
Benzaldehyde reacts with ammonia to form
benzaldehyde ammonia
urotropine
hydrobenzamide
ammonium chloride
Which of the following gives condensation with hydroxyl amine but does not undergo self condensation?
Methanal
Propanal
Acetone
Ethanal
The correct IUPAC name of [Co(NH3)3(NO2)3] is
Triammine trinitro-N cobalt (III)
Triammine trinitro-N cobalt (II)
Triammine cobalt (III) nitrite
Triammine trinitro-N cobaltate (III)
The replacement of diazonium group by fluorine is known as
Gattermann reaction
Sandmeyer reaction
Balz-Schiemann reaction
Etard reaction
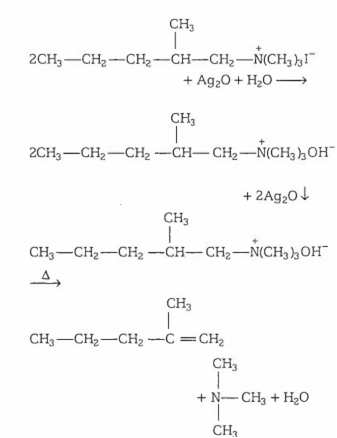
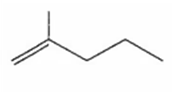
Identify the alkene that is produced in the following series of reactions

![]()
![]()
![]()
A.

Quaternary ammonium salt of alkyl amines, when reacts with silver oxide yield quaternary ammonium hydroxide. On strong heating, it decomposes into alkene, tert. amine and water. This reaction is known as Hofmann elimination reaction. Here elimination of H takes place from β-carbon having more hydrogen atoms.