 Multiple Choice Questions
Multiple Choice QuestionsPhenyl isocyanide is prepared from aniline by
Carbylamine reaction
Rosenmund's reaction
Koble's reaction
Reimer-Tiemann reaction
Gabriel's phthalimide synthesis can be used to prepare
ethanamine
N-methylmethanamine
benzene amine
N, N-dimethylmethanamine
Select the compound which on treatment with nitrous acid liberates nitrogen.
Nitroethane
Triethylamine
Diethylamine
Ethylamine
Positive carbylamine test is shown by
N, N-dimethylaniline
triethylamine
N-methylaniline
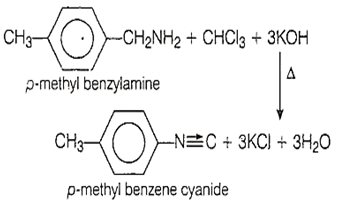
p-methylbenzylamine
D.
p-methylbenzylamine
Primary amines on heating with chloroform and alcoholic potassium hydroxide solution yield the corresponding isocyanides (carbylamines).
Ethanoic acid on heating with ammonia forms compound A which on treatment with bromine and sodium hydroxide gives compound B. Compound B on treatment with NaNO3/dil.HCl gives compound C. The compounds A, B and C respectively are
ethanamide, methanamine, methanol
propanamide, ethanamine, ethanol
N-ethylpropanamide, methaneisonitrile, methanamine
ethanamine, bromoethane, ethanediazoniumchloride
n-butylamine (l), diethylamine (II) and N, N-dimethylethylamine (III) have the same molar mass. The increasing order of their boiling point is
III < II < I
I < II < III
II < III < I
II < I < III
Choose the incorrect statement.
Primary amines show intermolecular hydrogen bonds
Tert-butylamine is a primary amine
Tertiary amines do not show intermolecular hydrogen bonds
Isopropylamine is a secondary amine
Amine that cannot be prepared by Gabriel phthalimide synthesis is
aniline
benzylamine
methylamine
iso-butylamine
