 Multiple Choice Questions
Multiple Choice QuestionsPhenyl isocyanide is prepared from aniline by
Carbylamine reaction
Rosenmund's reaction
Koble's reaction
Reimer-Tiemann reaction
Gabriel's phthalimide synthesis can be used to prepare
ethanamine
N-methylmethanamine
benzene amine
N, N-dimethylmethanamine
Select the compound which on treatment with nitrous acid liberates nitrogen.
Nitroethane
Triethylamine
Diethylamine
Ethylamine
Positive carbylamine test is shown by
N, N-dimethylaniline
triethylamine
N-methylaniline
p-methylbenzylamine
Ethanoic acid on heating with ammonia forms compound A which on treatment with bromine and sodium hydroxide gives compound B. Compound B on treatment with NaNO3/dil.HCl gives compound C. The compounds A, B and C respectively are
ethanamide, methanamine, methanol
propanamide, ethanamine, ethanol
N-ethylpropanamide, methaneisonitrile, methanamine
ethanamine, bromoethane, ethanediazoniumchloride
n-butylamine (l), diethylamine (II) and N, N-dimethylethylamine (III) have the same molar mass. The increasing order of their boiling point is
III < II < I
I < II < III
II < III < I
II < I < III
Choose the incorrect statement.
Primary amines show intermolecular hydrogen bonds
Tert-butylamine is a primary amine
Tertiary amines do not show intermolecular hydrogen bonds
Isopropylamine is a secondary amine
Amine that cannot be prepared by Gabriel phthalimide synthesis is
aniline
benzylamine
methylamine
iso-butylamine
A.
aniline
Only aliphatic primary amines can be prepared by Gabriel synthesis. Aniline cannot be prepared by this method because aryl halides (C6H5Cl or C6H5Br) do not undergo nucleophilic substitution with potassium phthalimide under ordinary conditions to give N-phenyl phthalimide (ie, cleavage of CX bond in haloarenes is quite difficult).
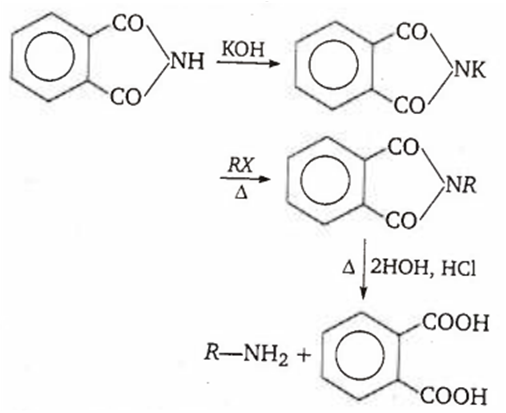
(RX = aliphatic halide)
