 Multiple Choice Questions
Multiple Choice QuestionsThe reaction of methyltrichloroacetate (Cl3CCO2Me) with sodium methoxide (NaOMe) generates
carbocation
carbene
carbanion
carbon radical
Identify the correct method for the synthesis of the compound shown below from the following alternatives.
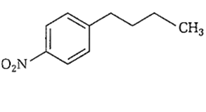
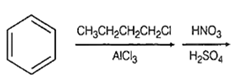
![]()
![]()
![]()
On Friedal Crafts acetylation, anisol yields
2-methoxyacetophenone
4-methoxyacetophenone
Both (a) and (b)
None of the above
Compound (A) when heated with ethyl magnesium iodide in dry ether forms an addition product, which on hydrolysis forms compound (B) Compound (B) on oxidation form 3-pantanone. Hence (A) and (B) are respectively
propanal, 3-pentanol
pentanol, 3-pentanol
ethanal, pentanol
acetone, 3-pentanol
The false statements among the following are
I. A primary carbocation is less stable than a tertiary carbocation.
II. A secondary propyl carbocation is less stable than allyl carbocation.
III. A tertiary free radical is more stable than a primary free radical.
IV. Isopropyl carbanion is more stable than ethyl carbanion.
I and II
II and III
I and IV
II and IV
D.
II and IV
Statements II and IV are wrong since 2° propyl carbocation is little more stable than allyl carbocation and ethyl carbanion is more stable than isopropyl carbanion.
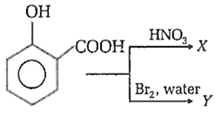
X and Y in the above given reaction are
picric acid, 2,4, 6-tribromophenol
5-nitrophenol acid, 5-bromosalicylic acid
o-nitrophenol, o-bromophenol
3, 5-dinitrosalicylic acid, 3,5-dibromosalicylic acid
