 Multiple Choice Questions
Multiple Choice QuestionsHydrogen peroxide when added to a solution of potassium permangnate acidified with sulphuric acid:
Acts as an oxidising agent
Acts as a reducing agent
Forms water only
Forms water only
Which of the following reactions depicts the reducing action of H2O2 ?
C6H6 + H2O2 C6H5OH + H2O
2I- + 2H+ + H2O2 I2 + H2O
2Mn + 6H+ +5H2O2 2Mn2+ + 5O2 + 8H2O
PbS + 4H2O2 PbSO4 + 4H2O
The density of SO2 at STP is 2.86 kg m3. Its density at 819°C and 2 atmospheres is:
0.715 kg m-3
1.43 kg m-3
2.86 kg m-3
4.26 kg m-3
Deuterium oxide is used in nuclear reactor as
source of -particle
source of deuteron
moderator
fuel
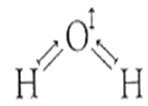
H2O is polar, whereas BeF2 is not because
electronegativity of F is greaterthan that of O.
H2O involves H-bonding, whereas BeF2 is a discrete molecule
H2O is angular and BeF2 is linear
H2O is linear and BeF2 is angular.
C.
H2O is angular and BeF2 is linear
Because of linear shape, dipole moments cancel each other in BeF2 (F Be F) and thus, it is non-polar, whereas H2O is V-shaped and hence, it is polar.
The correct order of solubility ofthe following compounds in water is
Ba(OH)2 < Mg(OH)2
BaCO3 > CaCO3
Ca(OH)2 Mg(OH)2
CaSO4 < MgSO4
