 Multiple Choice Questions
Multiple Choice QuestionsAn aqueous solution contains an unknown concentration of Ba2+. When 50 mL of a 1 M solution of Na2SO4 is added, BaSO4 just begins to precipitate. The final volume is 500 mL. The solubility product of BaSO4 is 1 x 10–10. What is the original concentration of Ba2+?
1.0 x 10–10 M
5 x 10–9 M
2x10–9 M
1.1 x 10–9M
Assuming the compounds to be completely dissociated in aqueous solution, identify the pair of the solutions that can be expected to be isotonic at the same temperature.
0.01 M urea and 0.01 M NaCl
0.02 M NaCl and 0.01 M Na2SO4
0.03 M NaCl and 0.02 M MgCl2
0.01 M sucrose and 0.02 M glucose
If and p are the vapour pressure of the pure solvent and solution and n1 and n2 are the moles of solute and solvent respectively in the solution then the correct relation between p and p° is
To observe an elevation of boiling point of 0.05C, the amount of a solute ( mol. wt. = 100) to be added to 100 g of water (Kb= 0.5) is
2g
0.5g
1g
0.75g
The measured freezing point depression for a 0.1 m aqueous CH3COOH solution is 0.19°C. The acid dissociation constant Ka at this concentration will be (Given, Kf the molal cryoscopic constant = 1.86 K kg mol-1)
4.76 × 10-5
4 × 10-5
8 × 10-5
2 × 10-5
58.4 g of NaCl and 180 g of glucose were separately dissolved in l000 mL of water. Identify the correct statement regarding the elevation of boiling point (b.p) of the resulting solutions.
NaCl solution will show higher elevation of boiling point
Glucose solution will show higher elevation of boiling point
Both the solutions will show equal elevation of boiling point
The boiling point elevation will be shown by neither of the solutions
Which of the following will show a negative deviation from Raoult's law?
Acetone-benzene
Acetone-ethanol
Benzene-methanol
Acetone-chloroform
D.
Acetone-chloroform
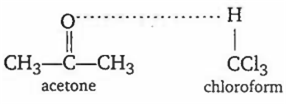
Acetone and chloroform will show a negative deviation due to their association after mixing.
A saturated solution of H2S in 0.1 M HCl at 25°C contains S2- ion concentration of 10-23 mol L-1. The solubility product of some sulphides are CuS = 10-44, FeS = 10-14, MnS = 10-15 , CdS = 10-25 . If 0.01 M solution of these salts in 1M HCl are saturated with H2S, which of these will be precipitated?
All
All except MnS
All except MnS and FeS
Only CuS
If the elevation in boiling point of a solution of 10 g of solute (mol. wt. = 100) in 100 g of water is the ebullioscopic constant of water is
10
100 Tb
