 Multiple Choice Questions
Multiple Choice QuestionsGiven: The mass of electron is 9.11 x 10-31 kg
Planck constant is 6.626 x 10-34 Js,
the uncertainty involved in the measurement of velocity within a distance of 0.1 A is:
5.79 x 106 ms-1
5.79 x 107 ms-1
5.79 x 108 ms-1
5.79 x 108 ms-1
The orientation of an atomic orbital is governed by:
azimuthal quantum number
spin quantum number
magnetic quantum number
magnetic quantum number
Which one is the wrong statement?
de-Broglie's wavelength is given by λ = h/mv,
where m = mass of the particle, v = group velocity of the particle
The uncertainty principle is 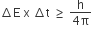
Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement
Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement
Which one is a wrong statement?
Total orbital angular momentum of electron in 's' orbital is equal to zero
An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers
The value of m for dz2 is zero
The electronic configuration of N atom is

Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is 1s2 2s2 2p3, the simplest formula for this compound is
Mg2X3
MgX2
Mg3X2
Mg2X
Among the following set of quantum numbers, the impossible set is
| n | l | m | s |
| 3 | 2 | -3 | -1/2 |
| n | l | m | s |
| 4 | 0 | 0 | 1/2 |
| n | l | m | s |
| 5 | 3 | 0 | -1/2 |
| n | l | m | s |
| 3 | 2 | -2 | 1/2 |
Match the type of series given in Column I with the wavelength range given in Column II and choose the correct option.
| Column I | Column II | ||
| A | Lyman | 1. | Ultraviolet |
| B. | Paschen | 2. | Near-infrared |
| C. | Balmer | 3. | Far Infrared |
| D. | Pfund | 4. | Visible |
| A | B | C | D |
| 1 | 2 | 4 | 3 |
| A | B | C | D |
| 4 | 3 | 1 | 2 |
| A | B | C | D |
| 3 | 1 | 2 | 4 |
| A | B | C | D |
| 4 | 3 | 2 | 1 |
The electrons identified by quantum numbers n and l, are as follows
n = 4, l = 0
n =3, l= 2
If we arrange them in order of increasing energy, i.e., from lowest to highest, the correct order is
IV<II<III<I
II<IV<I<III
I < III < II < IV
III < I < IV < II
What will be the number of waves formed by a Bohr electron in one complete revolution in its second orbit?
Three
Two
One
Zero
Match the particle with its characteristic
| Column I | Column II |
| A. -particle | p. Slow moving |
| B. Isobar | q. High penetration power |
| C. - ray | r. Same atomic mass |
| D. - particle | s. consists of electron |
A - p; B - r; C - q; D - s
A - p; B - q; C - r; D - s
A - r; B - s; C - p; D - q
A - s; B - r; C - p; D - q
