 Multiple Choice Questions
Multiple Choice QuestionsThe Langmuir adsorption isotherm is deduced using the assumption:
the adsorption takes place in multilayers
The adsorption sites are equivalent in their ability to adsorb the particles
The adsorbed molecules interact with each other
The adsorbed molecules interact with each other
A plot of log x/m versus log p for the adsorption of a gas on a solid gives a straight line with slope equal to:
-log k
n
1/n
1/n
On which of the following properties does the coagulating power of an ion depend?
The magnitude of the charge on the ion alone
Size of the ion alone
The sign of the charge on the ion alone
Both magnitude and sign of the charge on the ion
Given van der Waals constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
NH3
H2
CO2
O2
On the basis of Langmuir adsorption isotherm the amount of gas adsorbed at very high pressure.
Reaches a constant limiting value
Goes on increasing with pressure
Goes on decreasing with pressure
First increasing and then decreasing with pressure
If x is amount of adsorbate and m is amount of adsorbent, which of the following relations is not related to adsorption process?
Which of the following curves is in accordance with Freundlich adsorption isotherm?
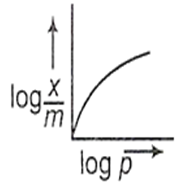


C.
Among all the given options, option c is correct.
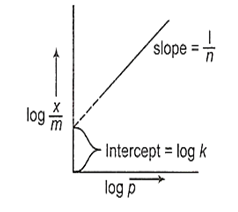
According to Freundlich Adsorption Isotherm, a graph between against log p is a straight line with slope equal to and intercept equal to log k.
In the presence of a catalyst, the heat evolved or absorbed during the reaction
increases
decreases
remains unchanged
may increase or decrease
