 Multiple Choice Questions
Multiple Choice QuestionsAcidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of
Cr
Cr2(SO3)3
CrSO4
Cr2(SO4)3
Point out the incorrect reaction from the following.
2Na2CrO4 + H+ Na2Cr2O7 + 2Na+ + H2O
4MnO2 + 4KOH + O2 4KMnO4 + 2H2O
2Mn + 5C2 + 16H+ 2Mn2+ + 10CO2 + 8H2O
Mn + 8H+ + 5Fe2+ 5Fe3+ + Mn2+ + 4H2O
On addition of small amount of KMnO4 to conc. H2SO4, a green oily compound is obtained which is highly explosive in nature. Identify the compound from the following
Mn2O7
MnO2
MnSO4
Mn2O3
White P reacts with caustic soda, the products are PH3 and NaH2PO2. This reaction is an example of
hydrolysis
reduction
disproportionation
neutralisation
The aqueous solution containing which one of the following ions will be colourless ? (Atomic no. Sc= 21, Fe= 26, Ti= 22, Mn = 25)
Sc3+
Fe2+
Ti3+
Mn2+
A.
Sc3+
21Sc = 1s2, 2s22p6, 3s23p63d1, 4s2
So, Sc3+ = 1s2, 2s22p6, 3s23p6
(It is colourless due to absence of unpaired electrons in d- stubshell)
26Fe = 1s2, 2s22p6, 3s23p63d6
Fe2+ = 1s2, 2s22p6, 3s23p63d6

(It is coloured due to presence of four unpaired electrons in d-subshell.)
22Ti = 1s2, 2s22p6, 3s23p63d2,4s2
Ti3+ = 1s2, 2s22p6, 3s2, 3s63d1

(It is coloured due to presence of one unpaired electron in d-subshell).
25Mn = 1s2, 2s22p6, 3s23p63d5, 4s2
Mn2+ = 1s2, 2s22p6, 3s23p63d5
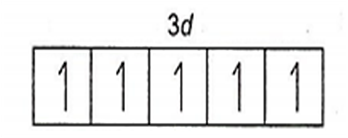
(It is coloured due to presence of five unpaired electrons in d-subshell).
The main reason for larger number of oxidation states exhibited by the actinides than the corresponding lanthanides, is
lesser energy difference between 5f and 6d orbitals than between 4f and 5d orbitals
larger atomic size of actinides than the lanthanide
more energy difference between 5f and 6d orbitals than between 4f and 5d orbitals
greater reactive nature of the actinides than the lanthanides
Cr is member of 3d transition series of atomic number 24. What will be its electronic configuration ?
3d64s2
3d54s2
3d44s2
3d54s1
The element 90Th232 belong to thorium series. Which of the following will act as the end product of the series?
82Pb208
82Bi209
82Pb206
82Pb207
