 Multiple Choice Questions
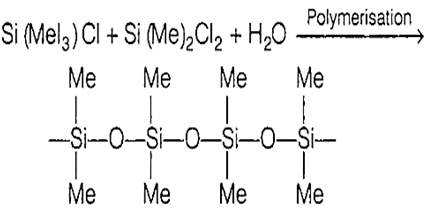
Multiple Choice QuestionsSilicon oil is obtained from the hydrolysis and polymerisation of
trimetylchlorosilane and dimethyldichlorosilane
trimethylchlorosilane and methyldichlorosilane
methyltrichlorosilane and dimethyldichlorosilane
triethylchlorosilane and dimethyldichlorosilane
A.
trimetylchlorosilane and dimethyldichlorosilane
Silicon oil in a polymer of trimethylchloro silane and dimethyldichloro silane. These are useful as broad spectrum antifoaming agents.
The orange solid on heating gives a colourless gas and a green solid which can be reduced to metal by aluminium powder. The orange and the green solids are, respectively
NH4Cr2O7 and Cr2O3
Na2Cr2O7 and Cr2O3
K2Cr2O7 and CrO3
(NH4)2CrO4 and CrO3
Match the following.
| List I | List II | ||
| (A) | Flespar | (I) | [Ag3SbS3] |
| (B) | Asbestos | (II) | Al2O3.H2O |
| (C) | Pyrargyrite | (III) | MgSO4.H2O |
| (D) | Diaspore | (IV) | KAlSi3O8 |
| (V) | CaMg3(SiO3)4 |
(A) (B) (C) (D)
IV V II I
(A) (B) (C) (D)
IV V I II
(A) (B) (C) (D)
IV I III II
(A) (B) (C) (D)
II V IV I
Diamond is hard because
all the four valence electrons are bonded to each carbon atoms by covalent bonds
it is a giant molecule
it is made up of carbon atoms
it cannot be burnt
Monosilane on coming in contact with air bums with a luminous flame producing vortex rings. These rings are of
SiO2
SiO
Si
H2SiO3
