Multiple Choice Questions
Multiple Choice QuestionsGraphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behaviour is that, graphite
is a non-crystalline substance
is an allotropic form of diamond
has molecules of variable molecular masses like polymers
has carbon atoms arranged in large plates of rings of strongly bound carbon atoms with weak interplate bonds
The structure of diborane (B2H6) contains
four 2C-2e- bonds and four 3C-2e- bonds
two 2C-2e- bonds and two 3C-3e- bonds
two 2C-2e- bonds and four 3C-2e- bonds
four 2C-2e- bonds and two 3C-2e- bonds
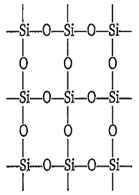
In silica (SiO2), each silicon atom is bonded to
two oxygen atoms
four oxygen atoms
one silicon and two oxygen atoms
one silicon and four oxygen atoms
B.
four oxygen atoms
The giant molecule of silicon dioxide or silica (SiO2) consists of continuous lattice of silicon and oxygen connected by covalent bonds. In silica each silicon atom is tetrahedrally surrounded by four oxygen atoms. Thus, there are no discrete SiO2 units. SiO2 is a network solid.
In silica, silicon has large size, so the 3p-orbitals of Si do not overlap effectively with 2p-orbitals of oxygen. Therefore, Si = O are not formed. The tetravalency of Si is satisfied by the formation of Si-O bonds, thus it is surrounded by four oxygen atoms.