 Multiple Choice Questions
Multiple Choice QuestionsThe gas produced by the passage of air over hot coke is
carbon monoxide
carbon dioxide
producer gas
water gas
The bonding in diborane (B2H6) can be described by
4 two centre - two electron bonds and 2 three - centre- two electron bonds
3 two centre - two electron bonds and 3 three - centre- two electron bonds
2 two centre - two electron bonds and 4 three centre - two electron bonds
4 two centre - two electron bonds and 4 two- centre- two electron bonds
A.
4 two centre - two electron bonds and 2 three - centre- two electron bonds
Diborane molecular formula B2H6.
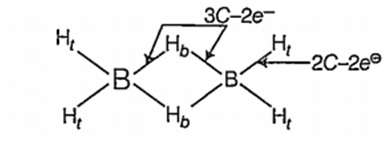
Diborane has 4 two centered two electron (2C-2e-) bonds and 2 three centre two electron (3C -2e-)bonds.
The correct statements among I to III regarding group 13 element oxides are,
(I) Boron trioxide is acidic.
(II) Oxides of aluminium and gallium are amphoteric.
(III) Oxides of indium and thallium are basic.
(I) and (II) only
(I), (II) and (III)
(I) and (III) only
(II) and (III) only
C60 an allotrope of carbon contains:
18 hexagons and 14 pentagons
20 hexagons and 12 pentagons
12 hexagons and 20 pentagons
16 hexagons and 16 pentagons
