 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following orders presents the correct sequence of the increasing basic nature of the given oxides?
Al2O3 < MgO < Na2O < K2O
MgO < K2O < Al2O3 < Na2O
Na2O < K2O < MgO < Al2O3
Na2O < K2O < MgO < Al2O3
Which of the following statements regarding sulphur is incorrect?
S2 molecule is paramagnetic.
The vapour at 200ºC consists mostly of S8 rings.
At 600ºC the gas mainly consists of S2 molecules.
At 600ºC the gas mainly consists of S2 molecules.
29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahl’s method and the evolved ammonia was absorbed in 20 mL of 0.1 M HCl solution. The excess of the acid required 15 mL of 0.1 M NaOH solution for complete neutralization. The percentage of nitrogen in the compound is
59.0
47.4
23.7
23.7
C.
23.7
m moles of HCl = 20 × 0.1 = 2
m moles of NaOH = 15 × 0.1 = 1.5
∴ m moles of HCl that consumed NH3 = 0.5
m moles of NH3 = 0.5
milligrams of N in NH3 = 0.5 × 14 = 7 mg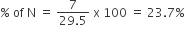
Which of the following reactions is an example of a redox reaction?
XeF4 + O2F2 → XeF6 + O2
XeF2 + PF5 → [XeF]+PF6–
XeF6 + H2O → XeOF4 + 2HF
XeF6 + H2O → XeOF4 + 2HF
The products obtained when chlorine gas reacts with cold and dilute aqueous NaOH are
ClO– and ClO3–
ClO2- and ClO3-
Cl– and ClO–
Cl– and ClO–
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
CH3OH
HCHO
CH3COCH3
CH3COCH3
In which of the following arrangements, the sequence is not strictly according to the property written against it?
CO2< SiO2< SnO2< PbO2: increasing oxidising power
HF< HCl < HBr < HI : increasing acid strength
NH3< PH3< AsH3< SbH3: increasing basic strength
NH3< PH3< AsH3< SbH3: increasing basic strength
Identify the incorrect statement among the following
Ozone reacts with SO2 to give SO3
Silicon reacts with NaOH(aq) in the presence of air to give Na2SiO3 and H2O
Cl2 reacts with excess of NH3 to give N2 and HCl
Cl2 reacts with excess of NH3 to give N2 and HCl
