 Multiple Choice Questions
Multiple Choice QuestionsWhat are the products formed when moist chlorine gas is reacted with hypo?
Na2SO4, S, HCl
Na2SO3, S, HCl
Na2S4O6, Na2SO3, HCl
Na2S4O6, NaCl, HCl
The hybridisation of Xe and the number of lone pairs of electrons on it in XeF6 are
sp3d2, 1
sp3d3, 2
sp3d2, 2
sp3d3, 1
0.16 g of an organic compound containing sulphur produces 0.233 g of BaSO4. Percentage of sulphur in the compound is
20
80
50
10
Which one of the following is not correct?
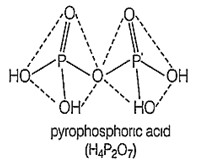
Pyrophosphoric acid is a tetrabasic acid.
Pyrophosphoric acid contains P-O-P linkage. Pyrophosphoric acrd contains two P-H bonds.
Pyrophosphoric acid contains two P-H bonds.
Orthophosphoric acd can be prepared by dissolvng P4O10 in water.
C.
Pyrophosphoric acid contains two P-H bonds.
In pyrophosphoric acid (H4P2O7) the phosphorus is bonded in tetrahedral manner with 4 sp3 bonds. It has +5 oxidation state and has four P-OH bonds, two P=O bonds and one P-O-P linkage. It can be represented as -
P4O10 + 6H2O → 4H3PO4
Liquid X is used in bubble chamber to detect neutral mesons and gamma photons. Then, X is
He
Ne
Kr
Xe
The catalyst and promoter respectively used in the Haber's process of industrial synthesis of ammonia are
Mo,V2O5
V2O5, Fe
Fe, Mo
Mo, Fe
Bond energy of Cl2, Br2 and I2 follow the order
Cl2 >Br2 > I2
Br2 >Cl2 > I2
I2 > Br2 >Cl2
I2 > Cl2 >Br2
Assertion (A): The boiling points of noble gases increases from He to Xe.
Reason (R): The interatomic van der Waals' attractive forces increases from He to Xe.
The correct answer is:
Both (A) and (R) are true, and (R) is the correct explanation of (A).
Both (A) and (R) are true, and (R) is not the correct explanation of (A).
(A) is true but (R) is not true.
(A) is not true but (R) is true.
