 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following figures represents the variation of the particle momentum and associated de-Broglie wavelength?
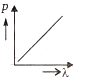

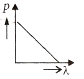
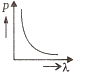
A and B are two metals with threshold frequencies 1.8 x 1014 Hz and 2.2 1014 Hz. Two identical photons of energy 0.825 eV each are incident on them. Then photoelectrons are emitted by (Take h = 6.6 x 10-34 J-s)
B alone
A alone
Neither A nor B
Both A and B
The emission electron is possible by
photoelectric effect
thermionic effect
both (a) and (b)
none of the above
An electron and a proton are possessing the same amount of kinetic energies. The de-Broglie wavelength is greater for :
electron
proton
both same
none of these
A.
electron
de-Broglie wavelength
Where h = Planck's constant
Electron and proton have the same kinetic energy,
So,
Mass of electron is less than that of a proton, so electron will have greater wavelength.
If the work function of a metal is 4.2 eV, the cut-off wavelength is :
8000 Å
7000 Å
1472 Å
2950 Å
The wavelength of light coming from a distant galaxy is found to be 0.5% greater than the wavelength of light coming from a stationary source. The galaxy is
coming towards earth with velocity of light
moving away from earth with velocity 1.5 x 106 m/sec
stationary with respect to earth
moving away from earth with velocity of light
Wave nature of light does not explain
Interference
Total internal reflection
Photo-electric effect
Refraction
The light of frequency v falls on a certain photoelectric substance of threshold frequency v0. The work function of the substance is
hv0
hv
h(v - v0)
The energy of a photon corresponding to the visible light of maximum wavelength is approximately
1 eV
1.6 eV
3.2 eV
7 eV
The rest mass of the photon is
zero
infinite
between zero and infinite
equal to that of electron
