 Multiple Choice Questions
Multiple Choice QuestionsThermodynamic processes are indicated in the following diagram.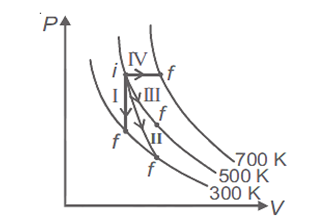
Match the following
| Column-1 | Column-2 |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
P → a, Q → c, R → d, S → b
P → c, Q → a, R → d, S → b
P → c, Q → d, R → b, S → a
P → c, Q → d, R → b, S → a
Carnot engine having an efficiency of 1/10 as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
1 J
90 J
99 J
99 J
A small sphere of radius 'r' falls from rest in a viscous liquid. As a result, heat is produced due to viscous force. The rate of production of heat when the sphere attains its terminal velocity, is proportional to
r3
r2
r4
r5
A sample of 0.1 g of water at 100°C and normal pressure (1.013 × 105 Nm–2) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample, is
104.3 J
208.7 J
84.5 J
42.2 J
The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is

2/5
2/3
2/7
1/3
The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is
26.8%
20%
12.5%
6.25%
p-V plots for two gases during the adiabatic process as shown in figure plots 1 and 2 should correspond respectively to

He and O2
O2 and He
He and Ar
O2 and N2
The temperature of source and sink of a heat engine are 127oC and 27oC, respectively. An inventor claims its efficiency to be 26%, then
It is impossible
It is possible with high probability
It is possible with low probability
Data are insufficient
A.
It is impossible
Efficiency of a heat engine is
Heat, 26% efficiency is impossible for a given heat engine.
Air is expanded from 50 litres to 150 litres at atmosphere the external work done is (1 atmospheric pressure =1)
200J
2000J
Helium at 270C has a volume 8 litres. it is suddenly compressed to a volume of 1 litre. The temperature of the gas will be
93270C
12000C
9270C
1080C
