 Multiple Choice Questions
Multiple Choice QuestionsAssertion (A) : NH2 group of aniline is ortho, para directing in electrophilic substitutions.
Reason (R) : -NH2 group stabilises the arenium ion formed by the ortho, para attack of the electrophile.
The correct answer is
Both (A) and (R) are correct, (R) is the correct explanation of (A).
Both (A) and (R) are correct, (R) is not the correct explanation of (A).
(A) is correct, but (R) is not correct.
(A) is not correct, but (R) is correct.
A.
Both (A) and (R) are correct, (R) is the correct explanation of (A).
The -NH2 group of aniline is a very strong electron donor (+M effect), hence it activates the benzene ring thoroughly and the electrophilic aromatic substitutions on the benzene ring are very easy to take place at ortho and para-positions.
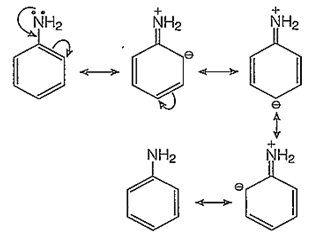
o-bromination:
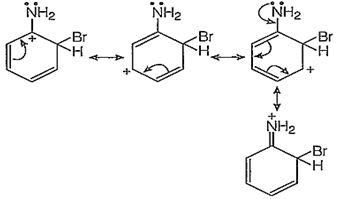
p-bromination :
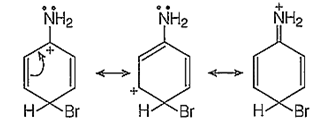
In addition to the usual resonating structures, that stabilizes the intermediate carbonium ion, the resonating strucutres formed by the interaction of lone pair electrons of nitrogen with the positively charged carbon of the ring also increase the stability of the carbonium ion formed durig the atttack of Br+ ion (electrophile) on o- and p-positions.
In Dumas method, 0.3 g of an organic compound gave 45 mL of nitrogen at STP. The percentage of nitrogen is
16.9
18.7
23.2
29.6
The IUPAC name of the following compound is-
(CH3)2CH-CH=CH-CH=CH(C2H5)-CH3
2, 7-dimethyl-3, 5-nonadiene
2, 7-dimethyl-2-ethylheptadene
2-methyl-7-ethyl-3, 5-octadiene
1, 1-dimethyl-6-ethyl-2, 4-heptadiene
Ferric ion forms a Prussian blue coloured precipitate due to formation of
K4[Fe(CN)6]
Fe4[Fe(CN)6]3
Fe(CNS)3
K3[Fe(CN)6]
Which of the following will show geometrical isomerism?
1-butene
2-butene
2-methyl propene
Propene
n-propyl alcohol and iso-propyl alcohol are examples of
position isomerism
chain isomerism
tautomerism
geometrical isomerism
The IUPAC name of the following compound is:
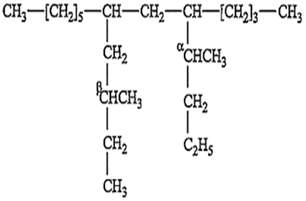
7 [β-methyl butyl]; 9 butyl tridecane
3 [β-ethyl butyl]; 9 ethyl tridecane
2 [β-ethyl ethenyl]; 8 propyl decane
None of the above
