 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compound possesses the C-H bond with the lowest bond dissociation energy?
Toluene
Benzene
n-pentane
2, 2-dimethyl propane
In the presence of HCl, H2S results the precipitation of group-2 elements but no group-4 elements during qualitative analysis. It is due to
higher concentration of S2-
higher concentration of H+
Lower concentration of S2-
Lower concentration of H+
Electrophile that participates in nitration of benzene is
NO+
NO
NO
NO
B.
NO
NO is an electrophile which takes part in nitration of benzene as

It is produced by taking mixture of conc. HNO3 and conc. H2SO4.
Which of the following is most likely to show optical isomerism ?
HC≡C--C≡CH
HC≡C--CH3
HC≡C--H
HC≡C-C (Cl) = CH2
Acetylene reacts with HCN in the presence of Ba(CN)2 to yield :
1, 1-dicyanoethane
1, 2-dicyanoethane
vinyl cyanide
None of the above
The following compound will undergo electrophilic substitution more readily than benzene:
nitrobenzene
benzoic acid
benzaldehyde
phenol
The correct statement about the compounds (a),(b)and (c) is :
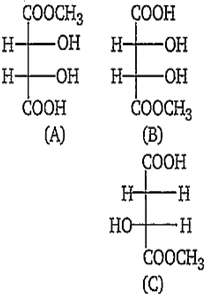
(A) and (B) are identical
(A) and (B) are diasteromers
(A) and (B) are not enantiomers
(A) and (B) are enantiomers
