 Multiple Choice Questions
Multiple Choice QuestionsConsider the following sets of quantum numbers. Which of the following setting is not permissible arrangement of electrons in an atom?
n = 4; l = 0; m = 0; s =
n = 5; l = 3; m = 0; s =
n = 3; l = 2; m = -2; s =
n = 3; l = 2; m = -3; s =
What is the effect of increasing pressure on the dissociation of PCl5 according to the equation?
PCl5 (g) PCl3 (g) + Cl2 (g)
Dissociation decreases
Dissociation increases
Dissociation does not change
None of the above
The ground state term symbol for an electronic state is governed by :
Heisenberg's principle
Hund's rule
Aufbau principle
Pauli exclusion principle
The correct set of quantum number for the unpaired electrons of chlorine atoms is
2, 1, -1, +1/2
2, 0, 0,+ 1/2
3, 1, 1, ±1/2
3, 0, 0, ±1/2
The number of electrons in the valence shell of sulphur in SF6 is :
12
10
8
11
A.
12
S has 6 electrons in its valance shell and it shares 6 electrons with 6 fluorine atoms, making a total of 12 electrons.
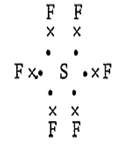
Rutherford's experiment on the scattering of α-particles showed for the first time that the atom has:
electrons
protons
nucleus
neutrons
