 Multiple Choice Questions
Multiple Choice QuestionsThe commercial production of methanol is done by
the catalytic reduction of CO in presence of ZnO, Cr2O3
the reaction of water vapour on CH4 at 900°C in presence of nickel catalys
the reaction offormaldehyde with LiAlH4
reaction of aqua KOH on HCHO
What is the IUPAC name of the follwoing compound?
![]()
3-methyl cyclo-1-butene-2-ol
4-methyl cyclobut-2-ene-1-ol
4-methyl cyclobut-1-ene-3-ol
2-methyl cyclo-3-butene-1-ol
Methoxy methane and ethanol are
functional isomers
chain isomers
optical isomers
geometrical isomers
The compound added to prevent chloroform to form phosgene gas (poisonous gas) is
CH3COOH
CH3OH
CH3COCH3
C2H5OH
The reaction C2H5ONa + BrC2H5 → C2H5-O-C2H5 + NaBr is called
Frankland reaction
Wurtz reaction
Williamson's synthesis
Cannizaro reaction
Diethyl ether on heating with conc. HI gives two moles of
ethyl iodide
ethanol
iodoform
methyl iodide
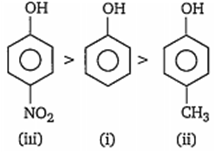
Correct acidic order of the following compounds is

i > ii> iii
iii> i> ii
ii> iii> i
i> iii> ii
B.
iii> i> ii
Presence of electron withdrawing group such as NO2 CHO etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxde ion while presence of electron releasing groups such as -CH3, -C2H5, destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compounds is
Identify Z in the following series
C2H5OH
CH2 = CH2
CH3-CH2OH
CH3-CH2-O-CH2-CH3
None of the above
The following compounds have identical molecular weight. Which would have the lowest boiling point?
2-butanol
2-methyl-1-propanol
1, 1-dimethyl ethanol
1-methoxypropane
