 Multiple Choice Questions
Multiple Choice QuestionsThe compound which gives turbidity immediately with Lucas reagent at room temperature is
butan-1-ol
butan-2-ol
2-methyl propan-2-ol
2-methyl propan-1-ol
The conversion of m-nitrophenol to resorcinol involves respectively
hydrolysis, diazotization and reduction
diazotization, reduction and hydrolysis
hydrolysis, reduction and diazotization
reduction, diazotization and hydrolysis
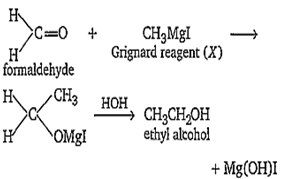
HCHO was treated with a reagent X. The product formed upon hydrolysis in the presence of an acid gave C2H5OH. The reagent X is
alcoholic KOH
alcoholic KCN
CH3MgI
aqueous KOH
C.
CH3MgI
The reagent X is CH3MgI.
Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence of dry ether?
diphenyl
propane
toulene
ethane
Power alcohol is a mixture of
80% petrol + 20% ethanol + small quantity of benzene
80% ethanol + 20% benzene + small quantity of petrol
50% Petrol + 50% ethanol + small quantity of benzene
80% petrol + 20% benzene + small quantity of ethanol
When CH2=CH-O-CH2-CH3 reacts with one mole of HI, one of the products formed is
ethane
ethanol
iodoethene
ethanal
0.44 g of a monohydric alcohol when added to methylmagnesium iodide in ether liberates at STP, 112 cm3 of methane. With PCC the same alcohol forms a carbonyl compound that answers silver mirror test. The monohydric alcohol is
H3C-CH(OH)-CH2-CH3
(CH3)3C-CH2OH
H3C-CH(OH)-CH2-CH2-CH3
(CH3)2CH-CH2OH
