Find the odd one out and explain your choice (Note: valency is not a criterion)
Al(OH)3, Pb(OH)2, Mg(OH)2, Zn(OH)2
Mg(OH)2 : It is basic while rest are amphoteric.
The diagram gives below is prepare Iron (III) chloride in the laboratory
i )What is substance ?
ii) what is the purpose of B ?
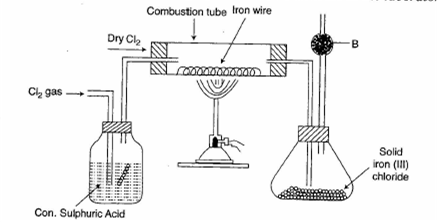
iii)why is iron (III) chloride to be stored in a closed container?
iv)write the equation for the reaction between iron and chlorine.
Match substance A to E listed below with the appropriate description:
(A ) sulphur
(B) silver chloride
( C) Hydrogen Chloride
( D ) Copper (II) sulphate
(E) Graphite.
A compound which is insoluble in cold water but soluble in excess of ammonia solution.
Identify the substance P, and R, based on the information given below:
The deliquescent salt P, turns yellow on dissolving in water, and gives a reddish brown precipitate with sodium hydroxide solution.]
Solution A is a strong
Solution B is a Weak acid
Solution C is a strong alkali
Which solution could be a solution of glacial acetic acid?
Identify the substance P, and R, based on the information given below:
The pale green solid R turns reddish brown on heating. Its aqueous solution gives a white precipitate with barium chloride solution. The precipitate is insoluble in mineral acids.
Find the odd one out and explain your choice (note:valency is not a criterion)
Formic acid, Nitric acid, acetic, propanoic acid.