CBSE
Class 10 Class 12
Unutilized or vacant spaces left after packing:
Tetrahedral void: A void between four touching spheres. Smaller in size.
Octahedral void: A void between six touching spheres. Bigger than tetrahedral voids.
Let the number of closed packed spheres be N, then:
The number of octahedral voids generated = N
The number of tetrahedral voids generated = 2N
Packing Efficiency:
Calculation of pacing efficiency in bcc (body-centered cubic) structure:
The packing efficiency can be calculated by the percent of space occupied by spheres present in a unit cell.
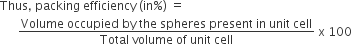
Since, there are 2 atoms present in the unit cell of bcc structure,
therefore, packing efficiency of bcc structure.


Calculation of pacing efficiency in hcp and ccp structure:
The packing efficiency can be calculated by the percent of space occupied by spheres present in a unit cell.
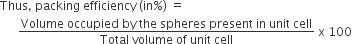
Since, there are 4 atoms present in the unit cell of hcp and ccp structure,
therefore, packing efficiency of hcp or ccp structure.

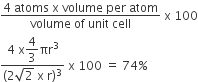
Calculation of pacing efficiency in simple cubic structure:
The packing efficiency can be calculated by the percent of space occupied by spheres present in a unit cell.
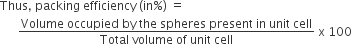
Since, there are 1 atoms present in the unit cell of simple cubic structure,
therefore, packing efficiency of simple cubic structure.

