 Long Answer Type
Long Answer TypeThe three classes of amines react differently with nitrous acid. Nitrous acid is very unstable and is generated only during the course of a reaction using NaNO2/HCl.
Primary amines have two hydrogens present on N-atoms.
Aromatic primary amine from diazonium salt with NaNO2/HCl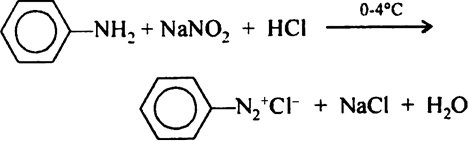
While Aliphatic primary amine form diazonium salt which is unstable and decompose to liberate N2 gas and form alcohol,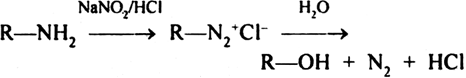
Secondary amines contain one hydrogen on N-atom. Both aliphatic and aromatic amines undergo electrophilic substitution reaction with HNO2 to form nitrosamines.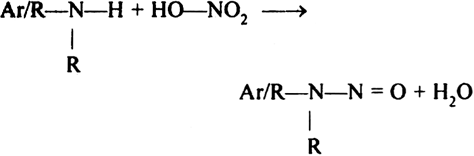
Tertiary amines have no H present on N-atom. Aliphatic tertiary amine form salt with HNO2 which is water soluble.
R3N + H—ONO → R3NH⊕®ONOΘ
Aromatic tertiary amine undergo electrophilic reaction at para position as the ring is highly activated by NR2 group.![]()
 Short Answer Type
Short Answer TypeExplain the following:
The amino group in ethylamine is basic whereas that in acetamide is not basic.
