 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compounds will be suitable for Kjeldah1’s method for nitrogen estimation?

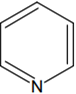


The increasing order of basicity of the following compounds is:

(d) < (b) < (a) < (c)
(a) < (b) < (c) < (d)
(b) < (a) < (c) < (d)
(b) < (a) < (d) < (c)
The compound that does not produce nitrogen gas by the thermal decomposition is
(NH4)2SO4
Ba(N3)2
(NH4)2Cr2O7
NH4NO2
The correct order of basicity of the following compounds is

1<2<3<4
1<2<4<3
2<1<3<4
4<3<2<1
C.
2<1<3<4
The correct order is 2<1<3<4. Since, more the number of 2° -NH- group, more is the basic nature.
(i) Thus, 3 and 4 are more basic than 1 and 2.
(ii) Between 3 and 4, 4 has one two NH2 group while 3 has only one NH2 group.
:. 4 is more basic than 3.
(iii) Between 1 and 2, 1 has one -NH2 group with no resonance, thus is more basic than 2.
Amongst the following compounds, the one(s) which readily react with ethanolic KCN.
Ethyl chloride
Chlorobenzene
Benzaldehyde
Salicylic acid
An amine C3H9N reacts with benzene sulphonyl chloride to form a white precipitate which is insoluble in aq. NaOH. The amine is


![]()

The reaction of aniline with chloroform under alkaline conditions leads to the formation of
phenylcyanide
phenylisonitrile
phenylcyanate
phenylisocyanate
When aniline is nitrated with nitrating mixture in ice cold condition, the major product obtained is
p-nitroaniline
2,4-dinitroaniline
o-nitroaniline
m-nitroaniline
The basicity of aniline is weaker in comparison to that of methyl amine due to
hyperconjugative effect of Me-group in MeNH2
resonance effect of phenyl group in aniline
lower molecular weight of methyl amine as compared to that of aniline
resonance effect of - NH2 group in MeNH2
